当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Three-component formal metathesis via remote activating strategy enabled (RASE) domino activation of two C(sp3)–O bonds in alkyl ethers
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-04 , DOI: 10.1039/d4qo00740a Dailin Zhuang 1 , Le Cheng 1 , Yichuan Yan 1 , Yuting Tang 1 , Zhenyang Wan 1 , Jiahao Gu 1 , Yuhong Lu 1 , Xinyao Li 2 , Ziyuan Li 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-06-04 , DOI: 10.1039/d4qo00740a Dailin Zhuang 1 , Le Cheng 1 , Yichuan Yan 1 , Yuting Tang 1 , Zhenyang Wan 1 , Jiahao Gu 1 , Yuhong Lu 1 , Xinyao Li 2 , Ziyuan Li 1
Affiliation
|
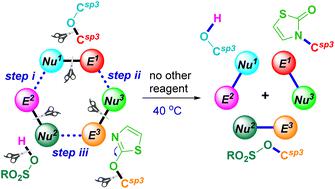
A remote activating strategy enabled (RASE) three-component formal metathesis among an alkyl benzyl ether, an alkyl aryl ether and a Brønsted acid is achieved through metal-free domino activation of two C(sp3)–O bonds in alkyl ethers under mild conditions, leading to dealkoxylative C(sp3)–N coupling products in good to excellent yields. With the use of in situ generated benzyl alkyl ether in this smooth metathesis, this chemistry offers an efficient strategy for direct site-selective introduction of pharmaceutically significant monocyclic thiazol-2(3H)-one scaffolds onto specific benzylic sites. A probable mechanism is also provided based on the results of control experiments and DFT calculations.
中文翻译:

通过远程激活策略 (RASE) 多米诺骨牌激活烷基醚中的两个 C(sp3)-O 键进行三组分形式复分解
通过两个 C(sp 3 )–O 的无金属多米诺骨牌激活,实现了烷基苄基醚、烷基芳基醚和布朗斯台德酸之间的远程激活策略 (RASE) 三组分形式复分解在温和的条件下,在烷基醚中形成键,产生脱烷氧基化的 C(sp 3 )–N 偶联产物,产率良好至优异。通过在这种顺利的复分解中使用原位生成的苄基烷基醚,该化学提供了一种有效的策略,可以将药学上重要的单环噻唑-2(3H)-one支架直接位点选择性地引入到特定的苄基位点上。根据控制实验和 DFT 计算的结果,还提供了可能的机制。
更新日期:2024-06-04
中文翻译:

通过远程激活策略 (RASE) 多米诺骨牌激活烷基醚中的两个 C(sp3)-O 键进行三组分形式复分解
通过两个 C(sp 3 )–O 的无金属多米诺骨牌激活,实现了烷基苄基醚、烷基芳基醚和布朗斯台德酸之间的远程激活策略 (RASE) 三组分形式复分解在温和的条件下,在烷基醚中形成键,产生脱烷氧基化的 C(sp 3 )–N 偶联产物,产率良好至优异。通过在这种顺利的复分解中使用原位生成的苄基烷基醚,该化学提供了一种有效的策略,可以将药学上重要的单环噻唑-2(3H)-one支架直接位点选择性地引入到特定的苄基位点上。根据控制实验和 DFT 计算的结果,还提供了可能的机制。






































 京公网安备 11010802027423号
京公网安备 11010802027423号