当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactive Crystallization Process of Li2CO3 from LiCl and Na2CO3 Mechanism and Modeling
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-06-01 , DOI: 10.1021/acs.iecr.3c04109
Teófilo A. Graber 1 , María E. Taboada 1 , Luis Cortés 1 , Eder Piceros 1, 2 , Gabriel Meruane 3 , Paola Aguilar 3
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-06-01 , DOI: 10.1021/acs.iecr.3c04109
Teófilo A. Graber 1 , María E. Taboada 1 , Luis Cortés 1 , Eder Piceros 1, 2 , Gabriel Meruane 3 , Paola Aguilar 3
Affiliation
|
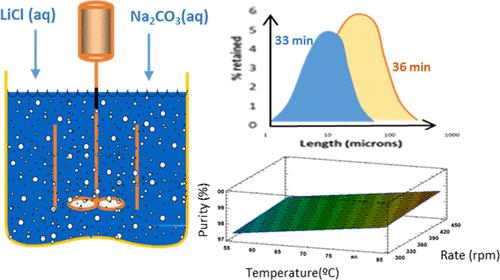
The main process to obtain lithium carbonate from brines is based on the reaction of lithium chloride with sodium carbonate. The present work seeks to find the most favorable conditions to produce lithium carbonate from industrial lithium chloride brines and sodium carbonate solutions. To this end, a 400 mL mechanically stirred batch crystallizer was employed to feed the sodium carbonate solution over the lithium brine in stoichiometric amounts. The reaction temperature (55–85 °C), stirring rate (300–400 rpm), and LiCl and Na2CO3 concentrations (1.54–2.53 mol/L) were varied. Primary homogeneous and heterogeneous nucleation zones of Li2CO3 were determined as functions of the degree of supersaturation and temperature. Crystal growth likely has a two-dimensional (2D) nucleation-mediated mechanism. A temperature increase enhances the Li2CO3 crystallization yield, slightly increases its purity, and generates a small particle size reduction. A rise in chemical concentration makes the crystallization yield and particle sizes increase, while it also produces lower purity percentages from the product. An increase in stirring rate causes a slight improvement in product purity, a small decrease in size, and no significant effect on crystallization yield.
中文翻译:

LiCl 和 Na2CO3 反应结晶 Li2CO3 机理和模型
从盐水中获取碳酸锂的主要过程是基于氯化锂与碳酸钠的反应。目前的工作旨在寻找从工业氯化锂盐水和碳酸钠溶液生产碳酸锂的最有利条件。为此,使用400mL机械搅拌间歇结晶器将化学计量的碳酸钠溶液供给到锂盐水上。反应温度 (55–85 °C)、搅拌速率 (300–400 rpm) 和 LiCl 和 Na 2 CO 3 浓度 (1.54–2.53 mol/L) 不同。 Li 2 CO 3 的初级均相和异相成核区被确定为过饱和度和温度的函数。晶体生长可能具有二维 (2D) 成核介导机制。温度升高提高了Li 2 CO 3 结晶产率,略微提高了其纯度,并产生了小幅粒径减小。化学浓度的增加使得结晶产率和粒度增加,同时也降低了产品的纯度百分比。搅拌速率的增加导致产品纯度略有提高,尺寸略有减小,对结晶收率没有显着影响。
更新日期:2024-06-01
中文翻译:

LiCl 和 Na2CO3 反应结晶 Li2CO3 机理和模型
从盐水中获取碳酸锂的主要过程是基于氯化锂与碳酸钠的反应。目前的工作旨在寻找从工业氯化锂盐水和碳酸钠溶液生产碳酸锂的最有利条件。为此,使用400mL机械搅拌间歇结晶器将化学计量的碳酸钠溶液供给到锂盐水上。反应温度 (55–85 °C)、搅拌速率 (300–400 rpm) 和 LiCl 和 Na 2 CO 3 浓度 (1.54–2.53 mol/L) 不同。 Li 2 CO 3 的初级均相和异相成核区被确定为过饱和度和温度的函数。晶体生长可能具有二维 (2D) 成核介导机制。温度升高提高了Li 2 CO 3 结晶产率,略微提高了其纯度,并产生了小幅粒径减小。化学浓度的增加使得结晶产率和粒度增加,同时也降低了产品的纯度百分比。搅拌速率的增加导致产品纯度略有提高,尺寸略有减小,对结晶收率没有显着影响。



































 京公网安备 11010802027423号
京公网安备 11010802027423号