当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Constructing asymmetric dual active sites through symbiotic effect for achieving efficient and selective photoreduction of CO2 to C2H4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-06-01 , DOI: 10.1039/d4ee01376j Yong Xu 1 , Ping Wang 1 , Man Zhang 1 , Weili Dai 1 , Yuxuan Xu 1 , Jian-Ping Zou 1 , Xubiao Luo 1, 2
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-06-01 , DOI: 10.1039/d4ee01376j Yong Xu 1 , Ping Wang 1 , Man Zhang 1 , Weili Dai 1 , Yuxuan Xu 1 , Jian-Ping Zou 1 , Xubiao Luo 1, 2
Affiliation
|
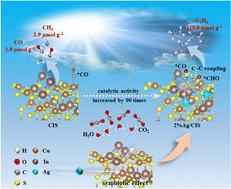
One of the biggest obstacles to the light-driven conversion of greenhouse gas CO2 into high value-added multi-carbon compounds is manipulating the C–C coupling reaction. Herein, we propose using a symbiotic effect to construct asymmetric dual active sites, namely doping CuInS2 (CIS) with single atomic Ag, accompanied by the formation of sulfur vacancies (Sv). Thus, Sv and the neighbouring In metal atom double sites are constructed, and CO2 photoreduction products undergo a transformation from C1 products to C2H4. Meanwhile, the doped Ag plays a role in capturing and transferring photogenerated electrons, improving the reduction rate. The generation rate of C2H4 is 53.8 μmol g−1 h−1 with a selectivity of 98.8%. Based on the number of transferred electrons, the catalytic activity of 2%Ag/CuInS2 (2%Ag/CIS) is 90 times that of CIS. Experimental and theoretical calculations verify that the key intermediates *CO and *CHO adsorbed on Sv and In sites, respectively, are propitious to promote the occurrence of the C–C coupling reaction owing to the reasonable distance, asymmetric electron distribution and distinct adsorption capacity. Accordingly, the findings provide a new strategy for designing asymmetric catalytic active sites for the selective photosynthesis of multi-carbon products from CO2.
中文翻译:

通过共生效应构建不对称双活性位点实现CO2高效选择性光还原为C2H4
将温室气体 CO 2 光驱动转化为高附加值多碳化合物的最大障碍之一是操纵 C-C 偶联反应。在此,我们提出利用共生效应构建不对称双活性位点,即用单原子Ag掺杂CuInS 2 (CIS),同时形成硫空位(S v ) 。由此,构建了S v 和邻近的In金属原子双位点,并且CO 2 光还原产物经历了从C 1 产物到C 2 H 4 。同时,掺杂的Ag起到捕获和转移光生电子的作用,提高还原率。 C 2 H 4 的生成率为 53.8 μmol g −1 h −1 ,选择性为 98.8%。根据转移电子数计算,2%Ag/CuInS 2 (2%Ag/CIS) 的催化活性是 CIS 的 90 倍。实验和理论计算验证了关键中间体*CO和*CHO分别吸附在S v 和In位点上,由于合理的距离、不对称性,有利于促进C-C偶联反应的发生。电子分布和独特的吸附能力。因此,这些发现为设计不对称催化活性位点以选择性光合作用CO 2 多碳产物提供了一种新策略。
更新日期:2024-06-01
中文翻译:

通过共生效应构建不对称双活性位点实现CO2高效选择性光还原为C2H4
将温室气体 CO 2 光驱动转化为高附加值多碳化合物的最大障碍之一是操纵 C-C 偶联反应。在此,我们提出利用共生效应构建不对称双活性位点,即用单原子Ag掺杂CuInS 2 (CIS),同时形成硫空位(S v ) 。由此,构建了S v 和邻近的In金属原子双位点,并且CO 2 光还原产物经历了从C 1 产物到C 2 H 4 。同时,掺杂的Ag起到捕获和转移光生电子的作用,提高还原率。 C 2 H 4 的生成率为 53.8 μmol g −1 h −1 ,选择性为 98.8%。根据转移电子数计算,2%Ag/CuInS 2 (2%Ag/CIS) 的催化活性是 CIS 的 90 倍。实验和理论计算验证了关键中间体*CO和*CHO分别吸附在S v 和In位点上,由于合理的距离、不对称性,有利于促进C-C偶联反应的发生。电子分布和独特的吸附能力。因此,这些发现为设计不对称催化活性位点以选择性光合作用CO 2 多碳产物提供了一种新策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号