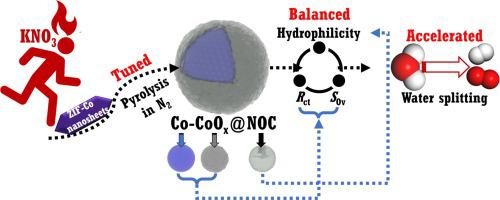
电化学催化水分解被认为是一种有前途的绿色制氢技术,需要针对阴极析氢反应(HER)和阳极析氧反应(OER)的高活性催化剂,特别是双功能催化剂。在这项工作中,通过ZIF-Co在少量KNO的辅助下热解制备了具有Co-CoO@N、O共掺杂碳(NOC)杂化纳米结构的双功能催化剂。 KNO 在调节 Co-CoO@NOC 杂化纳米结构的内部和外部结构方面发挥着重要作用。提出的定义为内部Co-CoO纳米颗粒单位表面积的氧空位(O)含量,并用电荷转移电阻()作为内部结构的描述符,而亲水性(或接触角)被用作外部结构的主要特征。和亲水性可以通过 KNO 的进料量很好地调节,适度的进料量是平衡 和亲水性的关键,并且进一步有利于加速 HER 和 OER 的特异性和内在活性。因此,双功能 HER/OER 特异性和内在活性通过 Co-CoO@NOC-0.025KNO 达到峰值,其中 KNO 的中等进料质量为 0.025 g,例如在 HER 和 OER 过电势下为 539.22 μA cm 和 129.83 μA cm分别为 250 和 350 mV,也分别对应于 6.653 和 0.801 s 的高周转频率。最佳催化剂还具有高表观活性,例如实现 500 mA cm 的高 HER 和 OER 电流密度,仅需要分别 273.1 和 382 mV 的低过电势。它在约 1.63 V 的电池电压下以 500 mA cm 的高电流密度促进整体水分解,显示出约 98 的高 HER 和 OER 法拉第效率。分别为 2 和 ~ 98.0%。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Pyrolysis of ZIF-Co assisted by a small amount of KNO3: Tuning of Co-CoOx@N, O co-doped carbon hybrid nanostructures for accelerating water splitting
Electrochemical catalytic water splitting is considered as a promising technique for green hydrogen production, and highly active catalysts toward cathodic hydrogen evolution reaction (HER) and anodic oxygen evolution reaction (OER) are required, especially bifunctional ones. In this work, the bifunctional catalysts with Co-CoO@N, O co-doped carbon (NOC) hybrid nanostructures have been prepared via pyrolysis of ZIF-Co with assistance of a small amount of KNO. KNO plays important roles in tuning the inner and the outer structures of Co-CoO@NOC hybrid nanostructures. The proposed , which is defined as the content of oxygen vacancy (O) per unit surface area of the inner Co-CoO nanoparticle, and charge transfer resistance () are used as the descriptors of the inner structures, while hydrophilicity (or contact angle) is applied as the main feature of the outer structures. , and hydrophilicity are well tuned by the fed mass of KNO, and a moderate fed mass is the key to balancing , and hydrophilicity and further is beneficial to accelerating specific and intrinsic activity for both HER and OER. Thus, the bifunctional HER/OER specific and intrinsic activity is peaked by Co-CoO@NOC-0.025KNO with a moderate fed mass of KNO of 0.025 g, such as of 539.22 μA cm and of 129.83 μA cm at the HER and OER overpotentials of 250 and 350 mV, respectively, also corresponding to high turnover frequencies of 6.653 and 0.801 s, respectively. The optimal catalyst also has high apparent activity, such as achieving high HER and OER current densities of 500 mA cm, only requiring low overpotentials of 273.1 and 382 mV, respectively. It promotes overall water splitting with a high current density of 500 mA cm at a cell voltage of ∼ 1.63 V, showing the high HER and OER Faradaic efficiencies of ∼ 98.2 and ∼ 98.0 %, respectively.


































 京公网安备 11010802027423号
京公网安备 11010802027423号