当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Action of KL-50, a Candidate Imidazotetrazine for the Treatment of Drug-Resistant Brain Cancers
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-05-30 , DOI: 10.1021/jacs.3c06483 Eric D Huseman 1 , Anna Lo 1 , Olga Fedorova 2, 3 , James L Elia 4 , Susan E Gueble 5 , Kingson Lin 1, 4, 5 , Ranjini K Sundaram 5 , Joonseok Oh 1, 6 , Jinchan Liu 1 , Fabian Menges 1, 7 , Matthew G Rees 8 , Melissa M Ronan 8 , Jennifer A Roth 8 , Victor S Batista 1 , Jason M Crawford 1, 7, 9 , Anna M Pyle 1, 2, 3 , Ranjit S Bindra 4, 5 , Seth B Herzon 1, 5, 10
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-05-30 , DOI: 10.1021/jacs.3c06483 Eric D Huseman 1 , Anna Lo 1 , Olga Fedorova 2, 3 , James L Elia 4 , Susan E Gueble 5 , Kingson Lin 1, 4, 5 , Ranjini K Sundaram 5 , Joonseok Oh 1, 6 , Jinchan Liu 1 , Fabian Menges 1, 7 , Matthew G Rees 8 , Melissa M Ronan 8 , Jennifer A Roth 8 , Victor S Batista 1 , Jason M Crawford 1, 7, 9 , Anna M Pyle 1, 2, 3 , Ranjit S Bindra 4, 5 , Seth B Herzon 1, 5, 10
Affiliation
|
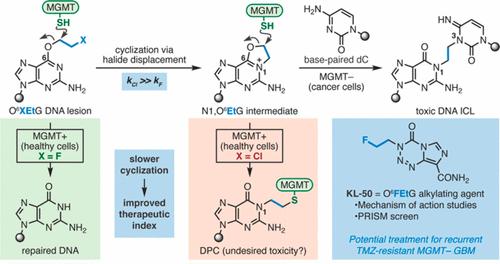
Aberrant DNA repair is a hallmark of cancer, and many tumors display reduced DNA repair capacities that sensitize them to genotoxins. Here, we demonstrate that the differential DNA repair capacities of healthy and transformed tissue may be exploited to obtain highly selective chemotherapies. We show that the novel N3-(2-fluoroethyl)imidazotetrazine “KL-50” is a selective toxin toward tumors that lack the DNA repair protein O6-methylguanine-DNA-methyltransferase (MGMT), which reverses the formation of O6-alkylguanine lesions. We establish that KL-50 generates DNA interstrand cross-links (ICLs) by a multistep process comprising DNA alkylation to generate an O6-(2-fluoroethyl)guanine (O6FEtG) lesion, slow unimolecular displacement of fluoride to form an N1,O6-ethanoguanine (N1,O6EtG) intermediate, and ring-opening by the adjacent cytidine. The slow rate of N1,O6EtG formation allows healthy cells expressing MGMT to reverse the initial O6FEtG lesion before it evolves to N1,O6EtG, thereby suppressing the formation of toxic DNA–MGMT cross-links and reducing the amount of DNA ICLs generated in healthy cells. In contrast, O6-(2-chloroethyl)guanine lesions produced by agents such as lomustine and the N3-(2-chloroethyl)imidazotetrazine mitozolomide rapidly evolve to N1,O6EtG, resulting in the formation of DNA–MGMT cross-links and DNA ICLs in healthy tissue. These studies suggest that careful consideration of the rates of chemical DNA modification and biochemical DNA repair may lead to the identification of other tumor-specific genotoxic agents.
中文翻译:

KL-50(一种治疗耐药脑癌的候选咪唑四嗪)的作用机制
异常的 DNA 修复是癌症的一个标志,许多肿瘤表现出 DNA 修复能力下降,从而使它们对基因毒素敏感。在这里,我们证明健康组织和转化组织的不同 DNA 修复能力可用于获得高度选择性的化疗。我们发现新型 N3-(2-氟乙基)咪唑四嗪“KL-50”是一种针对缺乏 DNA 修复蛋白 O 6 -甲基鸟嘌呤-DNA-甲基转移酶 (MGMT) 的肿瘤的选择性毒素,该蛋白可逆转 O 6 -烷基鸟嘌呤损伤。我们确定 KL-50 通过多步骤过程产生 DNA 链间交联 (ICL),包括 DNA 烷基化以产生 O 6 -(2-氟乙基)鸟嘌呤 (O 6 FEtG) 损伤,缓慢单分子置换氟化物以形成 N1 ,O 6 -乙醇鸟嘌呤(N1,O 6 EtG)中间体,并通过相邻胞苷开环。 N1,O 6 EtG 形成的缓慢速率使得表达 MGMT 的健康细胞能够在最初的 O 6 FEtG 病变发展为 N1,O 6 EtG 之前逆转它,从而抑制有毒 DNA-MGMT 交联的形成并减少DNA ICL 在健康细胞中生成。相反,由洛莫司汀和 N3-(2-氯乙基)咪唑四嗪 mitozolomide 等药物产生的 O 6 -(2-氯乙基)鸟嘌呤损伤迅速演化为 N1,O 6 EtG,导致 DNA-MGMT 交联的形成以及健康组织中的 DNA ICL。这些研究表明,仔细考虑化学 DNA 修饰和生化 DNA 修复的速率可能会导致其他肿瘤特异性基因毒性剂的鉴定。
更新日期:2024-05-30
中文翻译:

KL-50(一种治疗耐药脑癌的候选咪唑四嗪)的作用机制
异常的 DNA 修复是癌症的一个标志,许多肿瘤表现出 DNA 修复能力下降,从而使它们对基因毒素敏感。在这里,我们证明健康组织和转化组织的不同 DNA 修复能力可用于获得高度选择性的化疗。我们发现新型 N3-(2-氟乙基)咪唑四嗪“KL-50”是一种针对缺乏 DNA 修复蛋白 O 6 -甲基鸟嘌呤-DNA-甲基转移酶 (MGMT) 的肿瘤的选择性毒素,该蛋白可逆转 O 6 -烷基鸟嘌呤损伤。我们确定 KL-50 通过多步骤过程产生 DNA 链间交联 (ICL),包括 DNA 烷基化以产生 O 6 -(2-氟乙基)鸟嘌呤 (O 6 FEtG) 损伤,缓慢单分子置换氟化物以形成 N1 ,O 6 -乙醇鸟嘌呤(N1,O 6 EtG)中间体,并通过相邻胞苷开环。 N1,O 6 EtG 形成的缓慢速率使得表达 MGMT 的健康细胞能够在最初的 O 6 FEtG 病变发展为 N1,O 6 EtG 之前逆转它,从而抑制有毒 DNA-MGMT 交联的形成并减少DNA ICL 在健康细胞中生成。相反,由洛莫司汀和 N3-(2-氯乙基)咪唑四嗪 mitozolomide 等药物产生的 O 6 -(2-氯乙基)鸟嘌呤损伤迅速演化为 N1,O 6 EtG,导致 DNA-MGMT 交联的形成以及健康组织中的 DNA ICL。这些研究表明,仔细考虑化学 DNA 修饰和生化 DNA 修复的速率可能会导致其他肿瘤特异性基因毒性剂的鉴定。






























 京公网安备 11010802027423号
京公网安备 11010802027423号