Developmental Cell ( IF 10.7 ) Pub Date : 2024-05-31 , DOI: 10.1016/j.devcel.2024.05.010 Brian T. Do , Peggy P. Hsu , Sidney Y. Vermeulen , Zhishan Wang , Taghreed Hirz , Keene L. Abbott , Najihah Aziz , Joseph M. Replogle , Stefan Bjelosevic , Jonathan Paolino , Samantha A. Nelson , Samuel Block , Alicia M. Darnell , Raphael Ferreira , Hanyu Zhang , Jelena Milosevic , Daniel R. Schmidt , Christopher Chidley , Isaac S. Harris , Jonathan S. Weissman , Yana Pikman , Kimberly Stegmaier , Sihem Cheloufi , Xiaofeng A. Su , David B. Sykes , Matthew G. Vander Heiden
|
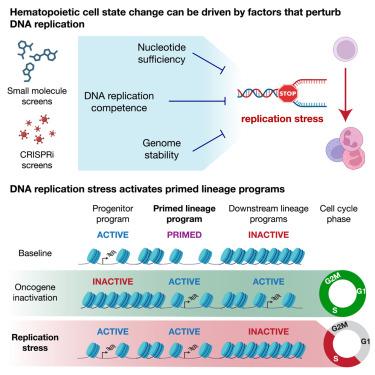
Control of cellular identity requires coordination of developmental programs with environmental factors such as nutrient availability, suggesting that perturbing metabolism can alter cell state. Here, we find that nucleotide depletion and DNA replication stress drive differentiation in human and murine normal and transformed hematopoietic systems, including patient-derived acute myeloid leukemia (AML) xenografts. These cell state transitions begin during S phase and are independent of ATR/ATM checkpoint signaling, double-stranded DNA break formation, and changes in cell cycle length. In systems where differentiation is blocked by oncogenic transcription factor expression, replication stress activates primed regulatory loci and induces lineage-appropriate maturation genes despite the persistence of progenitor programs. Altering the baseline cell state by manipulating transcription factor expression causes replication stress to induce genes specific for alternative lineages. The ability of replication stress to selectively activate primed maturation programs across different contexts suggests a general mechanism by which changes in metabolism can promote lineage-appropriate cell state transitions.
中文翻译:

核苷酸消耗通过诱导 DNA 复制应激促进细胞命运转变
细胞身份的控制需要协调发育程序与环境因素(例如营养物质的可用性),这表明扰乱新陈代谢可以改变细胞状态。在这里,我们发现核苷酸消耗和 DNA 复制应激驱动人类和小鼠正常和转化造血系统的分化,包括患者来源的急性髓系白血病 (AML) 异种移植物。这些细胞状态转变在 S 期开始,并且独立于 ATR/ATM 检查点信号、双链 DNA 断裂形成和细胞周期长度的变化。在分化被致癌转录因子表达阻断的系统中,尽管祖程序持续存在,但复制应激会激活引发的调控位点并诱导适合谱系的成熟基因。通过操纵转录因子表达来改变基线细胞状态会导致复制应激,从而诱导替代谱系特异的基因。复制应激在不同环境下选择性激活引发成熟程序的能力表明新陈代谢的变化可以促进谱系适当的细胞状态转变的一般机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号