当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
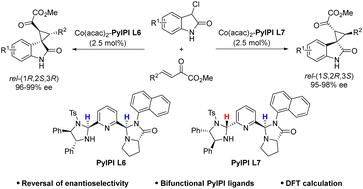
Reversal of enantioselectivity in cobalt(II)-catalyzed asymmetric Michael–alkylation reactions: synthesis of spiro-cyclopropane-oxindoles
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-05-31 , DOI: 10.1039/d4qo00681j Jun-Hao Zhang 1 , Wei-Jing Yang 1 , Ning Li 1 , Yin Tian 2 , Ming-Sheng Xie 1 , Hai-Ming Guo 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-05-31 , DOI: 10.1039/d4qo00681j Jun-Hao Zhang 1 , Wei-Jing Yang 1 , Ning Li 1 , Yin Tian 2 , Ming-Sheng Xie 1 , Hai-Ming Guo 1
Affiliation
|
Herein, an asymmetric Michael–alkylation reaction with a reversal of enantioselectivity catalyzed by chiral cobalt complexes is reported. Using 2.5 mol% of Co(acac)2-imidazolidine-pyrroloimidazolone pyridine as catalyst, a Michael–alkylation reaction between 3-chloro-oxindoles and β,γ-unsaturated-α-ketoesters was achieved, yielding chiral spiro-cyclopropane-oxindoles with a complete and controlled switch in stereoselectivity (up to 99% and −98% ee). The hydrogen bonding between the N–H group in the ligand and the oxyanion of the enolate derived from 3-chloro-oxindole is proposed to direct the reversal of enantioselectivity.
中文翻译:

钴(II)催化的不对称迈克尔烷基化反应中对映选择性的逆转:螺环丙烷羟吲哚的合成
在此,报道了由手性钴配合物催化的具有对映选择性逆转的不对称迈克尔烷基化反应。以2.5 mol%的Co(acac) 2 -咪唑烷-吡咯并咪唑酮吡啶为催化剂,3-氯-羟吲哚与β,γ-不饱和-α-酮酯发生迈克尔烷基化反应,得到手性化合物螺环丙烷羟吲哚具有完整且受控的立体选择性切换(高达 99% 和 -98% ee)。配体中的 N-H 基团与 3-氯-羟吲哚衍生的烯醇化物的氧阴离子之间的氢键被认为可以指导对映选择性的逆转。
更新日期:2024-06-05
中文翻译:

钴(II)催化的不对称迈克尔烷基化反应中对映选择性的逆转:螺环丙烷羟吲哚的合成
在此,报道了由手性钴配合物催化的具有对映选择性逆转的不对称迈克尔烷基化反应。以2.5 mol%的Co(acac) 2 -咪唑烷-吡咯并咪唑酮吡啶为催化剂,3-氯-羟吲哚与β,γ-不饱和-α-酮酯发生迈克尔烷基化反应,得到手性化合物螺环丙烷羟吲哚具有完整且受控的立体选择性切换(高达 99% 和 -98% ee)。配体中的 N-H 基团与 3-氯-羟吲哚衍生的烯醇化物的氧阴离子之间的氢键被认为可以指导对映选择性的逆转。






































 京公网安备 11010802027423号
京公网安备 11010802027423号