当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed Hydroxylation of Aryl Halides Using Hydroxypicolinamide Ligands
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-05-30 , DOI: 10.1021/acs.oprd.4c00108 Daniel W. Widlicka 1 , Robert A. Singer 1 , Ian Hotham 1 , David J. Bernhardson 1 , Samantha Grosslight 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-05-30 , DOI: 10.1021/acs.oprd.4c00108 Daniel W. Widlicka 1 , Robert A. Singer 1 , Ian Hotham 1 , David J. Bernhardson 1 , Samantha Grosslight 1
Affiliation
|
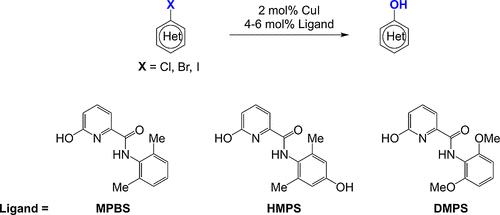
Hydroxylation of haloarenes is a fundamental transformation in synthetic organic chemistry. Hydroxypicolinamide ligands enable the efficient Cu-catalyzed hydroxylation of heteroaryl halides with a wide functional group tolerance. The Cu-MPBS system, originally designed for C–N coupling, enables the Cu-catalyzed hydroxylation of aryl bromides. A related derivative, Cu-HMPS, provides exceptional reactivity and purity for hydroxylation of aryl bromides, aryl iodides, and activated aryl chlorides. Ortho-activated substrates have shown exceptionally high reactivity and selectivity for Cu-catalyzed hydroxylation. More difficult aryl chlorides, substrates that require a higher activation temperature (120 °C), may be hydroxylated by the Cu-DMPS system that has superior intrinsic ligand stability. Reaction conditions may be tuned to target substrates through ligand, solvent, and base selection. Safe and robust processing conditions have been designed utilizing aqueous KOH, K2CO3, or K3PO4 in sulfolane or sulfolane and alcohol blends.
中文翻译:

使用羟基吡啶甲酰胺配体的铜催化芳基卤化物的羟基化
卤代芳烃的羟基化是有机合成化学中的一个基本转变。羟基吡啶甲酰胺配体能够实现杂芳基卤化物的有效铜催化羟基化,并具有广泛的官能团耐受性。 Cu-MPBS 系统最初设计用于 C-N 偶联,可实现铜催化芳基溴的羟基化。相关衍生物 Cu-HMPS 为芳基溴、芳基碘和活化芳基氯的羟基化提供了出色的反应性和纯度。邻位活化底物对铜催化的羟基化表现出极高的反应活性和选择性。更困难的芳基氯化物,需要更高活化温度(120°C)的底物,可以通过具有优异的内在配体稳定性的Cu-DMPS系统进行羟基化。可以通过配体、溶剂和碱基的选择来调整反应条件以适应目标底物。采用环丁砜或环丁砜中的 KOH、K 2 CO 3 或 K 3 PO 4 水溶液设计了安全可靠的加工条件和酒精混合物。
更新日期:2024-05-30
中文翻译:

使用羟基吡啶甲酰胺配体的铜催化芳基卤化物的羟基化
卤代芳烃的羟基化是有机合成化学中的一个基本转变。羟基吡啶甲酰胺配体能够实现杂芳基卤化物的有效铜催化羟基化,并具有广泛的官能团耐受性。 Cu-MPBS 系统最初设计用于 C-N 偶联,可实现铜催化芳基溴的羟基化。相关衍生物 Cu-HMPS 为芳基溴、芳基碘和活化芳基氯的羟基化提供了出色的反应性和纯度。邻位活化底物对铜催化的羟基化表现出极高的反应活性和选择性。更困难的芳基氯化物,需要更高活化温度(120°C)的底物,可以通过具有优异的内在配体稳定性的Cu-DMPS系统进行羟基化。可以通过配体、溶剂和碱基的选择来调整反应条件以适应目标底物。采用环丁砜或环丁砜中的 KOH、K 2 CO 3 或 K 3 PO 4 水溶液设计了安全可靠的加工条件和酒精混合物。






































 京公网安备 11010802027423号
京公网安备 11010802027423号