当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Detection mechanism and solvent effects of the 3-hydroxy-2-(4-(pyrrolidin-1-yl)phenyl)benzo[g]quinolin-4(1H)-one (PBQ) probe
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2024-05-29 , DOI: 10.1002/jccs.202400083 Meng Zhang 1 , Jin Li 1 , Jiaqi Li 1 , Yingmin Hou 1 , Yi Wang 1
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2024-05-29 , DOI: 10.1002/jccs.202400083 Meng Zhang 1 , Jin Li 1 , Jiaqi Li 1 , Yingmin Hou 1 , Yi Wang 1
Affiliation
|
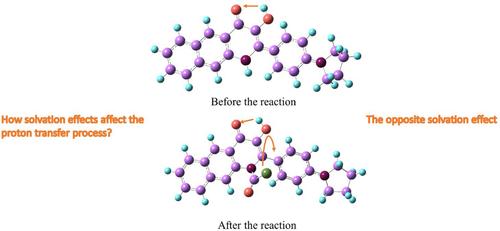
Hydroxy-2-(4-(pyrrolidin-1-yl)phenyl)benzo[g]quinolin-4(1H)-one (PBQ) is a ratiometric fluorescent probe based on excited-state intramolecular proton transfer (ESIPT). PBQ-1 is the reaction product following its exposure to phosgene. Density functional theory (DFT) and time dependent density functional theory (DFT) have been used to study the excited state dynamics of PBQ and PBQ-1 in different solvents. The results show that the reaction of PBQ with a transition from charge-transfer excitation to local excitation before and after the reaction. It becomes more difficult for PBQ in the excited state to transfer proton with increasing solvent polarity. The product PBQ-1 undergoes a molecular structure twist, and the angle of twisting decreases with increasing solvent polarity, resulting in a lower degree of rotational freedom of the hydroxyl group (5-OH) at the 5th carbon position, which makes it more susceptible to ESIPT reactions. Therefore, PBQ-1 is more susceptible to ESIPT as solvent polarity increases. Our theoretical calculations also elucidate the cause of the blue shift of PBQ fluorescence and the impact of the twisting intramolecular charge transfer phenomenon on the solvent effect. Furthermore, our study provides the theoretical guidance for the designing probe based on excited state intramolecular proton transfer.
中文翻译:

3-羟基-2-(4-(吡咯烷-1-基)苯基)苯并[g]喹啉-4(1H)-酮(PBQ)探针的检测机制和溶剂效应
Hydroxy-2-(4-(吡咯烷-1-基)苯基)苯并[g]喹啉-4(1H)-one (PBQ) 是一种基于激发态分子内质子转移 (ESIPT) 的比率荧光探针。 PBQ-1是暴露于光气后的反应产物。密度泛函理论(DFT)和时间相关密度泛函理论(DFT)已被用来研究PBQ和PBQ-1在不同溶剂中的激发态动力学。结果表明,PBQ的反应在反应前后经历了从电荷转移激发到局部激发的转变。随着溶剂极性的增加,激发态的 PBQ 转移质子变得更加困难。产品PBQ-1发生分子结构扭曲,扭曲角度随着溶剂极性的增加而减小,导致5号碳位羟基(5-OH)的旋转自由度降低,使其更容易受到影响。 ESIPT 反应。因此,随着溶剂极性的增加,PBQ-1 更容易受到 ESIPT 的影响。我们的理论计算还阐明了PBQ荧光蓝移的原因以及扭转分子内电荷转移现象对溶剂效应的影响。此外,我们的研究为基于激发态分子内质子转移的探针设计提供了理论指导。
更新日期:2024-05-29
中文翻译:

3-羟基-2-(4-(吡咯烷-1-基)苯基)苯并[g]喹啉-4(1H)-酮(PBQ)探针的检测机制和溶剂效应
Hydroxy-2-(4-(吡咯烷-1-基)苯基)苯并[g]喹啉-4(1H)-one (PBQ) 是一种基于激发态分子内质子转移 (ESIPT) 的比率荧光探针。 PBQ-1是暴露于光气后的反应产物。密度泛函理论(DFT)和时间相关密度泛函理论(DFT)已被用来研究PBQ和PBQ-1在不同溶剂中的激发态动力学。结果表明,PBQ的反应在反应前后经历了从电荷转移激发到局部激发的转变。随着溶剂极性的增加,激发态的 PBQ 转移质子变得更加困难。产品PBQ-1发生分子结构扭曲,扭曲角度随着溶剂极性的增加而减小,导致5号碳位羟基(5-OH)的旋转自由度降低,使其更容易受到影响。 ESIPT 反应。因此,随着溶剂极性的增加,PBQ-1 更容易受到 ESIPT 的影响。我们的理论计算还阐明了PBQ荧光蓝移的原因以及扭转分子内电荷转移现象对溶剂效应的影响。此外,我们的研究为基于激发态分子内质子转移的探针设计提供了理论指导。






























 京公网安备 11010802027423号
京公网安备 11010802027423号