当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mitochondrial RelA empowers mtDNA G-quadruplex formation for hypoxia adaptation in cancer cells
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2024-05-30 , DOI: 10.1016/j.chembiol.2024.05.003 Gui-Xue Tang 1 , Mao-Lin Li 1 , Cui Zhou 1 , Zhi-Shu Huang 2 , Shuo-Bin Chen 1 , Xiu-Cai Chen 3 , Jia-Heng Tan 2
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2024-05-30 , DOI: 10.1016/j.chembiol.2024.05.003 Gui-Xue Tang 1 , Mao-Lin Li 1 , Cui Zhou 1 , Zhi-Shu Huang 2 , Shuo-Bin Chen 1 , Xiu-Cai Chen 3 , Jia-Heng Tan 2
Affiliation
|
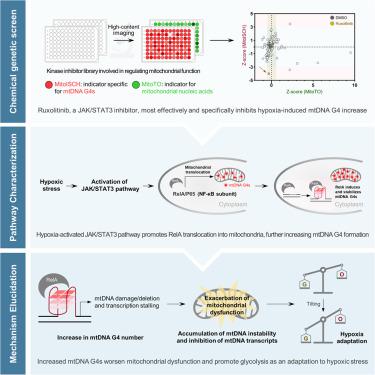
Mitochondrial DNA (mtDNA) G-quadruplexes (G4s) have important regulatory roles in energy metabolism, yet their specific functions and underlying regulatory mechanisms have not been delineated. Using a chemical-genetic screening strategy, we demonstrated that the JAK/STAT3 pathway is the primary regulatory mechanism governing mtDNA G4 dynamics in hypoxic cancer cells. Further proteomic analysis showed that activation of the JAK/STAT3 pathway facilitates the translocation of RelA, a member of the NF-κB family, to the mitochondria, where RelA binds to mtDNA G4s and promotes their folding, resulting in increased mtDNA instability, inhibited mtDNA transcription, and subsequent mitochondrial dysfunction. This binding event disrupts the equilibrium of energy metabolism, catalyzing a metabolic shift favoring glycolysis. Collectively, the results provide insights into a strategy employed by cancer cells to adapt to hypoxia through metabolic reprogramming.
中文翻译:

线粒体 RelA 使 mtDNA G 四链体形成能够适应癌细胞中的缺氧
线粒体 DNA (mtDNA) G-四链体 (G4) 在能量代谢中具有重要的调节作用,但它们的具体功能和潜在的调节机制尚未阐明。使用化学遗传学筛选策略,我们证明了 JAK/STAT3 通路是控制缺氧癌细胞中 mtDNA G4 动力学的主要调节机制。进一步的蛋白质组学分析表明,JAK/STAT3 通路的激活促进了 NF-κB 家族成员 RelA 转位到线粒体,在那里 RelA 与 mtDNA G4 结合并促进其折叠,导致 mtDNA 不稳定性增加,抑制 mtDNA 转录,并随后出现线粒体功能障碍。这种结合事件破坏了能量代谢的平衡,催化了有利于糖酵解的代谢转变。总的来说,这些结果为癌细胞通过代谢重编程适应缺氧的策略提供了见解。
更新日期:2024-05-30
中文翻译:

线粒体 RelA 使 mtDNA G 四链体形成能够适应癌细胞中的缺氧
线粒体 DNA (mtDNA) G-四链体 (G4) 在能量代谢中具有重要的调节作用,但它们的具体功能和潜在的调节机制尚未阐明。使用化学遗传学筛选策略,我们证明了 JAK/STAT3 通路是控制缺氧癌细胞中 mtDNA G4 动力学的主要调节机制。进一步的蛋白质组学分析表明,JAK/STAT3 通路的激活促进了 NF-κB 家族成员 RelA 转位到线粒体,在那里 RelA 与 mtDNA G4 结合并促进其折叠,导致 mtDNA 不稳定性增加,抑制 mtDNA 转录,并随后出现线粒体功能障碍。这种结合事件破坏了能量代谢的平衡,催化了有利于糖酵解的代谢转变。总的来说,这些结果为癌细胞通过代谢重编程适应缺氧的策略提供了见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号