Diabetologia ( IF 8.4 ) Pub Date : 2024-05-30 , DOI: 10.1007/s00125-024-06185-6 Selina Mäkinen 1, 2 , Sreesha Sree 1, 2 , Tuulia Ala-Nisula 3 , Henric Kultalahti 1, 2 , Peppi Koivunen 3 , Heikki A Koistinen 1, 2
|
Aims/hypothesis
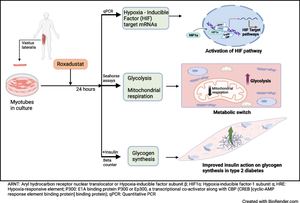
Hypoxia-inducible factor prolyl 4-hydroxylase (HIF-P4H) enzymes regulate adaptive cellular responses to low oxygen concentrations. Inhibition of HIF-P4Hs leads to stabilisation of hypoxia-inducible factors (HIFs) and activation of the HIF pathway affecting multiple biological processes to rescue cells from hypoxia. As evidence from animal models suggests that HIF-P4H inhibitors could be used to treat metabolic disorders associated with insulin resistance, we examined whether roxadustat, an HIF-P4H inhibitor approved for the treatment of renal anaemia, would have an effect on glucose metabolism in primary human myotubes.
Methods
Primary skeletal muscle cell cultures, established from biopsies of vastus lateralis muscle from men with normal glucose tolerance (NGT) (n=5) or type 2 diabetes (n=8), were treated with roxadustat. Induction of HIF target gene expression was detected with quantitative real-time PCR. Glucose uptake and glycogen synthesis were investigated with radioactive tracers. Glycolysis and mitochondrial respiration rates were measured with a Seahorse analyser.
Results
Exposure to roxadustat stabilised nuclear HIF1α protein expression in human myotubes. Treatment with roxadustat led to induction of HIF target gene mRNAs for GLUT1 (also known as SLC2A1), HK2, MCT4 (also known as SLC16A4) and HIF-P4H-2 (also known as PHD2 or EGLN1) in myotubes from donors with NGT, with a blunted response in myotubes from donors with type 2 diabetes. mRNAs for LDHA, PDK1 and GBE1 were induced to a similar degree in myotubes from donors with NGT or type 2 diabetes. Exposure of myotubes to roxadustat led to a 1.4-fold increase in glycolytic rate in myotubes from men with NGT (p=0.0370) and a 1.7-fold increase in myotubes from donors with type 2 diabetes (p=0.0044), with no difference between the groups (p=0.1391). Exposure to roxadustat led to a reduction in basal mitochondrial respiration in both groups (p<0.01). Basal glucose uptake rates were similar in myotubes from donors with NGT (20.2 ± 2.7 pmol mg−1 min−1) and type 2 diabetes (25.3 ± 4.4 pmol mg−1 min−1, p=0.4205). Treatment with roxadustat enhanced insulin-stimulated glucose uptake in myotubes from donors with NGT (1.4-fold vs insulin-only condition, p=0.0023). The basal rate of glucose incorporation into glycogen was lower in myotubes from donors with NGT (233 ± 12.4 nmol g−1 h−1) than in myotubes from donors with type 2 diabetes (360 ± 40.3 nmol g−1 h−1, p=0.0344). Insulin increased glycogen synthesis by 1.9-fold (p=0.0025) in myotubes from donors with NGT, whereas roxadustat did not affect their basal or insulin-stimulated glycogen synthesis. Insulin increased glycogen synthesis by 1.7-fold (p=0.0031) in myotubes from donors with type 2 diabetes. While basal glycogen synthesis was unaffected by roxadustat, pretreatment with roxadustat enhanced insulin-stimulated glycogen synthesis in myotubes from donors with type 2 diabetes (p=0.0345).
Conclusions/interpretation
Roxadustat increases glycolysis and inhibits mitochondrial respiration in primary human myotubes regardless of diabetes status. Roxadustat may also improve insulin action on glycogen synthesis in myotubes from donors with type 2 diabetes.
Graphical Abstract
中文翻译:

roxadustat 激活缺氧诱导因子途径可改善男性原代肌管的葡萄糖代谢
目标/假设
缺氧诱导因子脯氨酰 4-羟化酶 (HIF-P4H) 调节细胞对低氧浓度的适应性反应。抑制 HIF-P4H 会导致缺氧诱导因子 (HIF) 稳定并激活 HIF 通路,从而影响多种生物过程,从而拯救细胞免于缺氧。来自动物模型的证据表明 HIF-P4H 抑制剂可用于治疗与胰岛素抵抗相关的代谢紊乱,我们检查了罗沙司他(一种被批准用于治疗肾性贫血的 HIF-P4H 抑制剂)是否会对原发性糖尿病患者的葡萄糖代谢产生影响。人类肌管。
方法
原代骨骼肌细胞培养物是通过糖耐量正常 (NGT) ( n = 5) 或 2 型糖尿病 ( n = 8) 男性的股外侧肌活检建立的,并用罗沙司他治疗。通过定量实时 PCR 检测 HIF 靶基因表达的诱导。用放射性示踪剂研究葡萄糖摄取和糖原合成。使用 Seahorse 分析仪测量糖酵解和线粒体呼吸速率。
结果
暴露于 roxadustat 可稳定人肌管中核 HIF1α 蛋白的表达。 roxadustat治疗导致NGT供体肌管中诱导GLUT1 (也称为SLC2A1 )、 HK2 、 MCT4 (也称为SLC16A4 )和HIF-P4H-2 (也称为PHD2或EGLN1 )的HIF靶基因mRNA,患有 2 型糖尿病的捐赠者的肌管反应迟钝。 LDHA 、 PDK1和GBE1的 mRNA 在来自 NGT 或 2 型糖尿病捐赠者的肌管中被诱导至相似程度。肌管暴露于罗沙司他导致 NGT 男性肌管糖酵解率增加 1.4 倍( p = 0.0370),2 型糖尿病捐赠者肌管糖酵解率增加 1.7 倍( p = 0.0044),两者之间没有差异组( p = 0.1391)。暴露于 roxadustat 导致两组基础线粒体呼吸减少 ( p <0.01)。 NGT(20.2±2.7 pmol mg -1 min -1 )和2型糖尿病(25.3±4.4 pmol mg -1 min -1 , p = 0.4205)供体的肌管中的基础葡萄糖摄取率相似。 roxadustat 治疗可增强 NGT 捐赠者肌管中胰岛素刺激的葡萄糖摄取(与仅使用胰岛素的情况相比,为 1.4 倍, p = 0.0023)。 NGT 供体肌管中葡萄糖掺入糖原的基础率 (233 ± 12.4 nmol g −1 h −1 ) 低于 2 型糖尿病供体肌管 (360 ± 40.3 nmol g −1 h −1 , p =0.0344)。胰岛素使糖原合成增加 1。NGT 捐赠者的肌管中的糖原合成是 9 倍( p = 0.0025),而罗沙司他不影响其基础或胰岛素刺激的糖原合成。胰岛素使 2 型糖尿病捐献者肌管中的糖原合成增加了 1.7 倍( p = 0.0031)。虽然基础糖原合成不受罗沙司他的影响,但罗沙司他预处理增强了 2 型糖尿病供体肌管中胰岛素刺激的糖原合成 ( p = 0.0345)。
结论/解释
无论糖尿病状况如何,Roxadustat 都会增加人类原代肌管中的糖酵解并抑制线粒体呼吸。 Roxadustat 还可以改善胰岛素对 2 型糖尿病捐献者肌管中糖原合成的作用。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号