Medicinal Chemistry Research ( IF 2.6 ) Pub Date : 2024-05-28 , DOI: 10.1007/s00044-024-03240-0 Rakesh K. Bollikanda , Yana L. Esaulkova , Abburi Naga Pranathi , Devendra Nagineni , Nagaraju Chirra , Anna A. Muryleva , Pedapati Ravikumar , Vladimir V. Zarubaev , Srinivas Kantevari
|
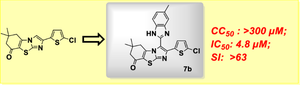
In this study, we present a series of 2-(5-chlorothiophen-2-yl)-1H-benzo-[d]-imidazol-2-yl)-6,7-dihydrobenzo-[d]-imidazo[2,1-b]thiazol-8(5H)-ones (7a–q) through a short, four-step process using readily available starting materials. The synthesis included crucial transformations such as the condensation of 1,3-cyclohexanediones with thiourea, cyclization with acetophenones, and the Vilsmeier-Haack-Arnold reaction. All the compounds were fully characterized and systematically screened for antibacterial, antifungal and antiviral activity. Among all, the compounds 7b (CC50: >1000 µM, IC50 = 4.8 µM, SI = > 63) and 7h (CC50: >1000 µM, IC50 = 5.6 µM, SI = > 54) with 3-methyl and 3-trifluoromethyl groups showed significant virus inhibitory activity against the pandemic influenza virus A/Puerto Rico/8/34 (H1N1) with high selectivity index values and favorable toxicity profiles. The compounds were also evaluated for antibacterial and antifungal activity, exhibited only moderate inhibition. Molecular docking studies have elucidated strong binding interactions with the viral target, the RNA polymerase PB1-PB2 subunits of influenza A virus. ADMET profiles highlighted encouraging drug-like properties and positioned 2-(5-chlorothiophen-2-yl)-1H-benzo-[d]-imidazol-2-yl)-6,7-dihydrobenzo-[d]-imidazo [2,1-b]thiazol-8(5H)-one as a promising candidate for further development as antiviral therapeutics.
中文翻译:

苯并[d]咪唑-6,7-二氢苯并[d]咪唑[2,1-b]噻唑-8(5H)-酮的设计和合成作为有效的抗感染剂
在这项研究中,我们提出了一系列2-(5-氯噻吩-2-基)-1H-苯并-[d]-咪唑-2-基)-6,7-二氢苯并-[d]-咪唑[2, 1-b]噻唑-8(5H)-酮 (7a–q),使用现成的起始原料,通过简短的四步工艺制备。合成过程包括关键的转化,例如 1,3-环己二酮与硫脲的缩合、苯乙酮的环化以及 Vilsmeier-Haack-Arnold 反应。所有化合物均经过全面表征并系统筛选其抗菌、抗真菌和抗病毒活性。其中,化合物 7b(CC50:>1000 µM,IC50 = 4.8 µM,SI = > 63)和 7h(CC50:>1000 µM,IC50 = 5.6 µM,SI = > 54)具有 3-甲基和 3-三氟甲基各组对大流行性流感病毒 A/Puerto Rico/8/34 (H1N1) 显示出显着的病毒抑制活性,具有高选择性指数值和良好的毒性特征。还评估了这些化合物的抗菌和抗真菌活性,仅表现出中等抑制作用。分子对接研究阐明了与病毒靶标(甲型流感病毒的 RNA 聚合酶 PB1-PB2 亚基)的强结合相互作用。 ADMET 简介强调了令人鼓舞的类似药物特性,并定位了 2-(5-氯噻吩-2-基)-1H-苯并-[d]-咪唑-2-基)-6,7-二氢苯并-[d]-咪唑[2 ,1-b]thiazol-8(5H)-one 作为进一步开发抗病毒疗法的有希望的候选者。































 京公网安备 11010802027423号
京公网安备 11010802027423号