当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
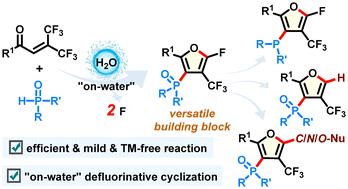
“On-water” defluorinative cyclization of trifluoromethyl enones with phosphine oxides: synthesis of polysubstituted furans
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-05-29 , DOI: 10.1039/d4qo00659c Man-Hang Feng 1 , Shu-Ji Gao 1 , Xiao-Ying Li 1 , Mengtao Ma 2 , Zhi-Liang Shen 1 , Xue-Qiang Chu 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-05-29 , DOI: 10.1039/d4qo00659c Man-Hang Feng 1 , Shu-Ji Gao 1 , Xiao-Ying Li 1 , Mengtao Ma 2 , Zhi-Liang Shen 1 , Xue-Qiang Chu 1
Affiliation
|
The unique properties at the macroscopic phase boundary between water and insoluble reactants have the potential to enhance reaction rates and selectivity. In this work, a mild, transition-metal-free, and efficient defluorinative cyclization of readily available trifluoromethyl enones with phosphine oxides is developed in a pure water solution, involving a sequence of intermolecular defluorophosphorylation and intramolecular defluoroheterocyclization. A variety of polysubstituted furans featuring C1-fluorine, C2-trifluoromethyl, and C3-phosphoryl groups could be synthesized in good yields with excellent tolerance toward various functional groups. Moreover, these furans could be readily transformed into other structurally valuable furan derivatives. The utilization of water not only serves as an environmentally friendly solvent but also contributes to excellent reaction selectivity and selective C–F bond activation.
中文翻译:

三氟亚甲基酮与氧化膦的“水上”脱氟环化:多取代呋喃的合成
水和不溶性反应物之间宏观相界的独特性质有可能提高反应速率和选择性。在这项工作中,在纯水溶液中开发了一种容易获得的三氟亚甲基烯酮与氧化膦的温和、无过渡金属且高效的脱氟环化,涉及一系列分子间脱氟磷酸化和分子内脱氟杂环化。可以以良好的产率合成各种具有 C1-氟、C2-三氟甲基和 C3-磷酰基的多取代呋喃,并对各种官能团具有优异的耐受性。此外,这些呋喃可以很容易地转化为其他结构上有价值的呋喃衍生物。水的利用不仅作为一种环境友好的溶剂,而且有助于优异的反应选择性和选择性C-F键活化。
更新日期:2024-06-03
中文翻译:

三氟亚甲基酮与氧化膦的“水上”脱氟环化:多取代呋喃的合成
水和不溶性反应物之间宏观相界的独特性质有可能提高反应速率和选择性。在这项工作中,在纯水溶液中开发了一种容易获得的三氟亚甲基烯酮与氧化膦的温和、无过渡金属且高效的脱氟环化,涉及一系列分子间脱氟磷酸化和分子内脱氟杂环化。可以以良好的产率合成各种具有 C1-氟、C2-三氟甲基和 C3-磷酰基的多取代呋喃,并对各种官能团具有优异的耐受性。此外,这些呋喃可以很容易地转化为其他结构上有价值的呋喃衍生物。水的利用不仅作为一种环境友好的溶剂,而且有助于优异的反应选择性和选择性C-F键活化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号