当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimization of LiF dissolution with Al2(SO4)3 and its application to lithium extraction by fluorination of α-spodumene
Hydrometallurgy ( IF 4.8 ) Pub Date : 2024-05-18 , DOI: 10.1016/j.hydromet.2024.106336 Gustavo D. Rosales , Alexander C. Resentera , Gretel J. Fino , Eliana G. Pinna , Mario H. Rodriguez
Hydrometallurgy ( IF 4.8 ) Pub Date : 2024-05-18 , DOI: 10.1016/j.hydromet.2024.106336 Gustavo D. Rosales , Alexander C. Resentera , Gretel J. Fino , Eliana G. Pinna , Mario H. Rodriguez
|
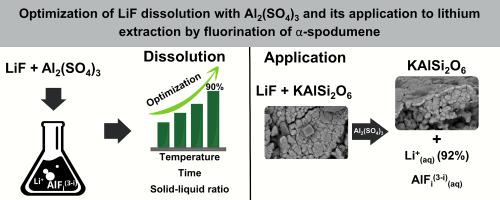
Numerous Li extraction methods from minerals and e-waste have been reported in the literature. Among them, direct fluorination processes appear to be a viable alternative due to their high lithium extraction efficiencies (>90%) as LiF. However, a drawback is the low water solubility of LiF, which requires acids for its separation and to obtain other commercial lithium salts. An interesting alternative for dissolving salts with low solubility is through the formation of coordination complexes. In this case, aluminum forms highly stable soluble complexes with the F anion, such as AlF, AlF, AlF, AlF, AlF, AlF.
中文翻译:

Al2(SO4)3 溶解 LiF 的优化及其在 α-锂辉石氟化提锂中的应用
文献报道了许多从矿物和电子废物中提取锂的方法。其中,直接氟化工艺似乎是一种可行的替代方案,因为其与 LiF 一样具有较高的锂提取效率 (>90%)。然而,LiF的缺点是水溶性低,需要酸来分离并获得其他商业锂盐。溶解低溶解度盐的一种有趣的替代方法是通过形成配位络合物。在这种情况下,铝与F阴离子形成高度稳定的可溶性络合物,例如AlF、AlF、AlF、AlF、AlF、AlF。
更新日期:2024-05-18
中文翻译:

Al2(SO4)3 溶解 LiF 的优化及其在 α-锂辉石氟化提锂中的应用
文献报道了许多从矿物和电子废物中提取锂的方法。其中,直接氟化工艺似乎是一种可行的替代方案,因为其与 LiF 一样具有较高的锂提取效率 (>90%)。然而,LiF的缺点是水溶性低,需要酸来分离并获得其他商业锂盐。溶解低溶解度盐的一种有趣的替代方法是通过形成配位络合物。在这种情况下,铝与F阴离子形成高度稳定的可溶性络合物,例如AlF、AlF、AlF、AlF、AlF、AlF。






























 京公网安备 11010802027423号
京公网安备 11010802027423号