Cell Chemical Biology ( IF 6.6 ) Pub Date : 2024-05-28 , DOI: 10.1016/j.chembiol.2024.05.001 Valentina Rossio 1 , Joao A Paulo 1 , Xinyue Liu 1 , Steven P Gygi 1 , Randall W King 1
|
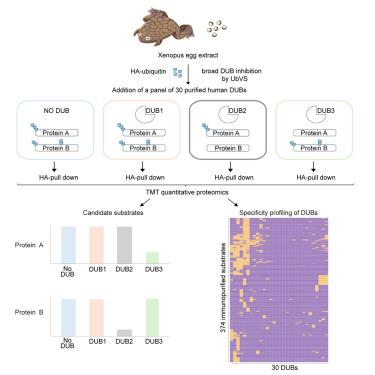
Deubiquitylating enzymes (DUBs) remove ubiquitin from proteins thereby regulating their stability or activity. Our understanding of DUB-substrate specificity is limited because DUBs are typically not compared to each other against many physiological substrates. By broadly inhibiting DUBs in Xenopus egg extract, we generated hundreds of ubiquitylated proteins and compared the ability of 30 DUBs to deubiquitylate them using quantitative proteomics. We identified five high-impact DUBs (USP7, USP9X, USP36, USP15, and USP24) that each reduced ubiquitylation of over 10% of the isolated proteins. Candidate substrates of high-impact DUBs showed substantial overlap and were enriched for disordered regions, suggesting this feature may promote substrate recognition. Other DUBs showed lower impact and non-overlapping specificity, targeting distinct non-disordered proteins including complexes such as the ribosome or the proteasome. Altogether our study identifies candidate DUB substrates and defines patterns of functional redundancy and specificity, revealing substrate characteristics that may influence DUB-substrate recognition.
中文翻译:

去泛素化酶针对内源生成的泛素-蛋白质缀合物的特异性分析
去泛素化酶 (DUB) 可去除蛋白质中的泛素,从而调节其稳定性或活性。我们对 DUB 底物特异性的理解是有限的,因为 DUB 通常不会与许多生理底物进行比较。通过广泛抑制非洲爪蟾卵提取物中的 DUB,我们生成了数百种泛素化蛋白质,并使用定量蛋白质组学比较了 30 种 DUB 去泛素化它们的能力。我们确定了 5 种高影响 DUB(USP7、USP9X、USP36、USP15 和 USP24),每种都降低了超过 10% 的分离蛋白质的泛素化。高影响力 DUB 的候选底物显示出大量重叠,并且富含无序区域,表明这一特征可能会促进底物识别。其他 DUB 显示出较低的影响和非重叠特异性,针对不同的非无序蛋白质,包括核糖体或蛋白酶体等复合物。总之,我们的研究确定了候选 DUB 底物并定义了功能冗余和特异性的模式,揭示了可能影响 DUB 底物识别的底物特征。































 京公网安备 11010802027423号
京公网安备 11010802027423号