Cell ( IF 45.5 ) Pub Date : 2024-05-28 , DOI: 10.1016/j.cell.2024.04.046
Fangyu Liu 1 , Anat Levit Kaplan 2 , Jesper Levring 3 , Jürgen Einsiedel 4 , Stephanie Tiedt 4 , Katharina Distler 4 , Natalie S Omattage 3 , Ivan S Kondratov 5 , Yurii S Moroz 6 , Harlan L Pietz 3 , John J Irwin 2 , Peter Gmeiner 4 , Brian K Shoichet 2 , Jue Chen 7
|
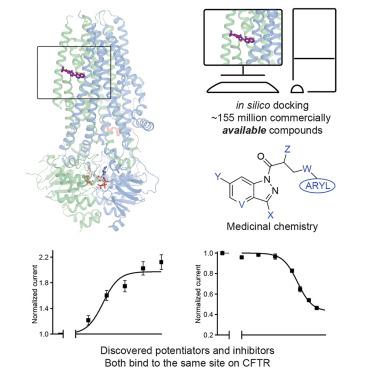
The cystic fibrosis transmembrane conductance regulator (CFTR) is a crucial ion channel whose loss of function leads to cystic fibrosis, whereas its hyperactivation leads to secretory diarrhea. Small molecules that improve CFTR folding (correctors) or function (potentiators) are clinically available. However, the only potentiator, ivacaftor, has suboptimal pharmacokinetics and inhibitors have yet to be clinically developed. Here, we combine molecular docking, electrophysiology, cryo-EM, and medicinal chemistry to identify CFTR modulators. We docked ∼155 million molecules into the potentiator site on CFTR, synthesized 53 test ligands, and used structure-based optimization to identify candidate modulators. This approach uncovered mid-nanomolar potentiators, as well as inhibitors, that bind to the same allosteric site. These molecules represent potential leads for the development of more effective drugs for cystic fibrosis and secretory diarrhea, demonstrating the feasibility of large-scale docking for ion channel drug discovery.
中文翻译:

基于结构的 CFTR 增强剂和抑制剂的发现
囊性纤维化跨膜电导调节器(CFTR)是一个重要的离子通道,其功能丧失会导致囊性纤维化,而其过度激活会导致分泌性腹泻。改善 CFTR 折叠(校正剂)或功能(增强剂)的小分子已在临床上可用。然而,唯一的增强剂ivacaftor的药代动力学欠佳,抑制剂尚未临床开发。在这里,我们结合分子对接、电生理学、冷冻电镜和药物化学来鉴定 CFTR 调节剂。我们将约 1.55 亿个分子对接至 CFTR 的增效剂位点,合成了 53 个测试配体,并使用基于结构的优化来识别候选调节剂。这种方法发现了与相同变构位点结合的中纳摩尔增效剂和抑制剂。这些分子代表了开发更有效的囊性纤维化和分泌性腹泻药物的潜在先导,证明了大规模对接离子通道药物发现的可行性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号