Journal of Fluorescence ( IF 2.6 ) Pub Date : 2024-05-28 , DOI: 10.1007/s10895-024-03764-z Javed Hussain Shah 1 , Shahzad Sharif 1 , Muhammad Shahbaz 1 , Bilal Riaz 1 , Sundas Shahzad 1 , Onur Şahin 2 , Khurram Shahzad Munawar 3 , Hijaz Ahmad 4, 5 , Essam A Al-Ammar 6
|
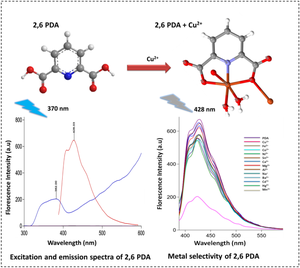
Copper metal is third most abundant trace element in human body. Determination of Cu (II) ions is a burning topic in field of environment protection and food safety because of its significant impact on ecosystem. In this study, 2,6-pyridine dicarboxylic acid (PDA) has been explored as “turn-off” florescent probe for florescent detection of Cu (II) ions. This sensor showed highly selective complexing ability towards Cu (II) ions. Addition of aqueous solution of Cu (II) ions remarkably quenched the fluorescence intensity of PDA while, on contrary, there was no any prominent fluorescence quenching interference on addition of various metal ions. The binding mode of PDA and Cu (II) ions was determined as stoichiometry of 1:1 and it was further confirmed by single crystal XRD analysis. Mechanisms of static and dynamic quenching were confirmed by stern–volmer plot. Limit of detection (LOD) and limit of quantification (LOQ) for Cu (II) ions was calculated as 3.6 µM and 1.23 µM respectively, which is far below the acceptable value (31.5µM) according to the World Health Organization. The use of the sensor for detection of Cu (II) ions in real samples in aqueous media was also performed.
Graphical Abstract
中文翻译:

吡啶-2,6-二甲酸作为一种简便且高选择性的“关闭”荧光化学传感器,用于检测水介质中的 Cu (II) 离子
铜金属是人体内含量第三丰富的微量元素。 Cu(II)离子的测定因其对生态系统的重大影响而成为环境保护和食品安全领域的热门话题。在本研究中,2,6-吡啶二甲酸 (PDA) 被探索作为“关闭”荧光探针,用于荧光检测 Cu (II) 离子。该传感器对 Cu (II) 离子表现出高度选择性络合能力。 Cu(II)离子水溶液的添加显着猝灭了PDA的荧光强度,而添加各种金属离子则没有明显的荧光猝灭干扰。 PDA与Cu(II)离子的结合模式确定为1:1的化学计量,并通过单晶XRD分析进一步证实。静态和动态猝灭机制通过斯特恩-沃尔默图得到证实。 Cu (II) 离子的检测限 (LOD) 和定量限 (LOQ) 分别计算为 3.6 µM 和 1.23 µM,远低于世界卫生组织规定的可接受值 (31.5 µM)。还使用该传感器检测水介质中实际样品中的 Cu (II) 离子。

































 京公网安备 11010802027423号
京公网安备 11010802027423号