Journal of Ecology ( IF 5.3 ) Pub Date : 2024-05-27 , DOI: 10.1111/1365-2745.14340 Laura Méndez 1 , Christopher D. Barratt 1 , Walter Durka 1, 2 , W. Daniel Kissling 3 , Wolf L. Eiserhardt 4, 5 , William J. Baker 4 , Vonona Randrianasolo 6 , Renske E. Onstein 1, 7
|
1 INTRODUCTION
More than 90% of woody plant species in tropical rainforests rely on frugivores (i.e., fruit-eating and seed-dispersing animals) for their seed dispersal (Jordano, 2000). For these plants, most long-distance dispersal events are provided by large-bodied frugivores (i.e. megafrugivores—the subset of largest animal species that have fruit as the main part of their diet in a given ecosystem; Moleón et al., 2020), that are able to ingest large fruits and seeds (e.g. ‘megafaunal’ fruits, larger than 4 cm in diameter; Guimarães et al., 2008) and move them across long distances given their large home ranges (Pires et al., 2018). Megafrugivores, such as the African elephant (Loxodonta africana), thereby connect their food plant populations, leading to increased gene flow and low speciation rates of plants with megafaunal fruits (McConkey et al., 2018; Onstein et al., 2017). By influencing population connectivity and gene flow, seed dispersal is crucial for the regional-scale genetic structure of plant populations (Browne et al., 2018).
During the last 125,000 years, extinction rates of large-bodied vertebrates have increased globally due to human impact (Smith et al., 2018). The extirpation of megafrugivores from ecosystems may have had cascading effects on the seed dispersal and thus connectivity of plant populations, especially for plants carrying large fruits that cannot be dispersed by the remaining smaller-bodied frugivores in the ecosystem (Janzen & Martin, 1982). Frugivore extinctions may therefore limit plant seed dispersal and gene flow, leading to high genetic differentiation (Giombini et al., 2017; Pérez-Méndez et al., 2016), with major consequences for the capacity of vertebrate-dispersed plants to track climate change (Fricke et al., 2022). Such historical interactions with now extinct megafrugivores may also have left imprints on current species and populations. For example, the turnover (i.e. beta diversity) of palm species across assemblages in Madagascar was shaped—at least partly—by historical co-occurrences with now extinct megafrugivores, predominantly in the western region of the island (Méndez, Viana, et al., 2022a). Similarly, the Neotropical distribution of palm fruit sizes was best explained by considering past—now extinct—megafrugivores (Lim et al., 2020). However, whether historical interactions with now extinct megafrugivores have also affected plant population genetics remains largely unknown.
In Madagascar, the decline of megafauna began approximately 1000 years ago, primarily due to increasing human impact (Crowley, 2010). Hunting and a transition to herding and farming, changed landscapes and megafauna habitats, ultimately leading to the extirpation of all megafauna (Godfrey et al., 2019; Li et al., 2020). Some of these extinct megafaunal species, such as giant lemurs (e.g. Pachylemur spp. and Archaeolemur spp.), elephant birds (e.g. Aepyornis spp. and Vorombe spp.) and giant tortoises (Aldabrachelys spp.), were probably fruit-eaters (Godfrey et al., 2004; Pedrono et al., 2013). Interestingly, fossil pollen suggests that the extinction of endemic megafauna in Madagascar coincided with a gradual decline in abundance of trees relying on megafrugivores for seed dispersal (Domic et al., 2021). However, there is no direct evidence of any plant species extinction in response to the extinction of its megafrugivore interaction partners. Instead, it has been suggested that several plant species that were adapted to seed dispersal by megafrugivores have persisted in today's ecosystems due to domestication by humans (Kistler et al., 2015), secondary seed dispersal by smaller-bodied frugivores (Blanco et al., 2019), or other, non-biotic forms of dispersal (e.g. via rivers; Guimarães et al., 2008).
Environmental and landscape-related factors may have shaped plant population genetics through non-frugivory-related dispersal processes (Jiang et al., 2019; Sexton et al., 2014; Siepielski et al., 2017). Environmental suitability (i.e. the extent to which a specific geographic area provides the appropriate conditions for a species to survive and thrive) can act as either a barrier or a corridor for dispersal (McRae, 2006; Wang & Bradburd, 2014), thereby influencing genetic differentiation of plant populations. For example, rivers in Madagascar acted as barriers to dispersal, leading to high genetic structure in leafless vanilla orchids (Vanilla spp.; Andriamihaja et al., 2021), while they acted as dispersal corridors for a megafruited tree, Eligmocarpus cynometroides (Fabaceae), allowing its spread across several biomes (Devey et al., 2013). Furthermore, forest cover, particularly of riparian forests, played an important role in connecting forest patches and structuring genetic diversity of an endemic tree in Madagascar (i.e. Noronhia spinifolia, Salmona et al., 2022).
Human activities have also been a major determinant of present-day genetics of plant populations (Smith et al., 2020). For example, fragmentation of ecosystems by human-made infrastructures, such as settlements or road networks, may limit the movement of large vertebrates (e.g. frugivorous mammals) across the landscape, leading to dispersal limitation of plants with large vertebrate-dispersed fruits (Tucker et al., 2021). However, by moving plant seeds, humans may also facilitate long-distance dispersal events (Wichmann et al., 2008), potentially decreasing genetic differentiation among plant populations (Arredondo et al., 2018; Bullock et al., 2018). Additionally, unpaved roads can function as seed corridors for plants, as some terrestrial mammals select them for faecal marking, facilitating seed dispersal along road verges (Suárez-Esteban et al., 2013). Human activities may therefore either increase gene flow among plant populations or disrupt gene flow, depending on the context.
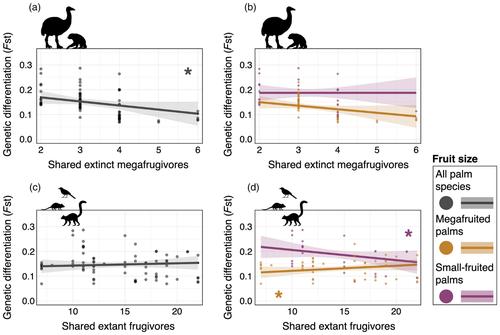
Here, we assessed whether patterns resulting from dispersal services provided by Madagascar's past megafrugivores can still be detected in the genomes of current palm (Arecaceae) populations. We focussed on palms because several species have been identified as ‘anachronisms’ in Madagascar's ecosystems due to their megafaunal fruit sizes (>4 cm in length, hereafter referred to as megafruits) that seem maladapted to dispersal by the current frugivore pool (Albert-Daviaud et al., 2020). We selected species from the western part of the island, where megafrugivorous animals were possibly most abundant in the past (Crowley et al., 2011). These savanna vertebrate-dispersed palm species can be classified in three fruit size classes: large megafruits (30 cm in average length—Borassus madagascariensis), medium-sized megafruits (5.5 cm—Hyphaene coriacea, 4.4 cm—Bismarckia nobilis) and small fruits (<4 cm; 1.3 cm—Chrysalidocarpus madagascariensis [previously Dypsis madagascariensis, Eiserhardt et al., 2022]; Table 1). The relatively small fruits of C. madagascariensis can still be dispersed by extant frugivores (e.g. Eulemur macaco; Adany et al., 1994), whereas the megafruits of the other three species are too large to be swallowed and dispersed by any native extant frugivore on Madagascar (Perry & Hartstone-Rose, 2010). Furthermore, the palms with medium-sized megafruits (H. coriacea and B. nobilis) are highly used by humans for house construction, basketry or food (Rakotoarinivo et al., 2020). Human-mediated dispersal may thus have contributed to the persistence and genetics of these species during the last 1000 years. Finally, the three megafruit species belong to the tribe Borasseae (B. madagascariensis, H. coriacea and B. nobilis) and are dioecious, with male and female individuals separated, while C. madagascariensis is monoecious (male and female flowers on the same individual). Little is known about the pollinators of these palms, but flowers are relatively small and inconspicuous, suggesting a wide range of insect pollinators (Henderson, 1986).
| Fruit length (cm) | Number of populations | Mean Fst (SD) | Mean M-among-pops (SD) | Mean M-within-pops (SD) | |
|---|---|---|---|---|---|
| Borassus madagascariensis | 30 | 3 | 0.192 (0.035) | 0.031 (0.007) | 0.938 (0.015) |
| Hyphaene coriacea | 5.5 | 7 | 0.129 (0.034) | 0.025 (0.001) | 0.847 (0.008) |
| Bismarckia nobilis | 4.4 | 8 | 0.125 (0.039) | 0.029 (0.019) | 0.798 (0.047) |
| Chrysalidocarpus madagascariensis | 1.3 | 7 | 0.188 (0.061) | 0.025 (0.004) | 0.847 (0.015) |
We hypothesize (H1) that historical long-distance dispersal events by megafrugivores facilitated gene flow between populations, thereby leaving imprints on the current genetics of megafruit palm populations. Specifically, we expect that palm populations that historically shared more megafrugivore species were more connected through frequent megafrugivore-mediated seed dispersal and gene flow, leading to greater genetic similarity (i.e. lower genetic differentiation) than populations that shared fewer megafrugivore species. In contrast, genetic differentiation of populations of the small-fruited palm are expected to have been primarily shaped by past and ongoing dispersal by extant smaller-bodied frugivores, with a higher number of shared extant frugivores leading to lower among-population genetic differentiation. Furthermore, we hypothesize that landscape-related features such as forests, rivers and environmental suitability (H2) and humans (H3) have contributed to the genetic differentiation and recent migration rates of palm populations, possibly by replacing seed dispersal services of megafrugivores after their extinction. Specifically, we expect that forest cover, environmental suitability and river density have served as corridors for gene flow, thereby reducing genetic differentiation and increasing migration rates among populations. Similarly, human population density and road density may have increased population connectivity by facilitating dispersal among plant populations, either directly through movement of plant material and/or indirectly, by providing corridors for extant frugivores that move along road verges. Alternatively, humans and roads may have fragmented landscapes and reduced population connectivity, thereby increasing population differentiation and decreasing migration rates among populations.
To test these hypotheses, we integrated palm population-level genomic data with past inferences of megafrugivore distributions, current frugivore distributions, landscape and human impact variables. We applied linear mixed effect models to disentangle the frugivory-related, landscape-related and human-related drivers of palm population genetic differentiation and recent migration rates. Our results provide novel insights into how past interactions with megafrugivores may have influenced the genetics of plants on Madagascar.
中文翻译:

马达加斯加棕榈树中过去巨型食果动物介导的扩散的基因组特征
1 简介
热带雨林中 90% 以上的木本植物物种依靠食果动物(即吃水果和传播种子的动物)来传播种子(Jordano,2000)。对于这些植物来说,大多数长距离扩散事件是由大型食果动物提供的(即巨型食果动物——在给定生态系统中以水果作为其饮食的主要部分的最大动物物种的子集;Moleón 等人,2020),它们能够摄取大型水果和种子(例如直径大于 4 厘米的“巨型动物”水果;Guimarães 等人,2008 年),并因其活动范围大而将它们长距离移动(Pires 等人,2018 年)。巨型食果动物,例如非洲象(Loxodonta africana),从而将它们的食用植物种群连接起来,导致巨型动物果实植物的基因流增加和物种形成率降低(McConkey等人,2018年;Onstein等人,2017年)。通过影响种群连通性和基因流动,种子传播对于植物种群的区域规模遗传结构至关重要(Browne et al., 2018)。
在过去 125,000 年中,由于人类影响,全球大型脊椎动物的灭绝率有所增加(Smith 等,2018)。巨型食果动物从生态系统中的灭绝可能对种子传播产生级联效应,从而影响植物种群的连通性,特别是对于携带大型果实的植物,这些果实无法被生态系统中剩余的较小体型食果动物传播(Janzen&Martin,1982)。因此,食果动物灭绝可能会限制植物种子传播和基因流动,导致高度遗传分化(Giombini et al., 2017;Pérez-Méndez et al., 2016),对脊椎动物分散植物追踪气候变化的能力产生重大影响(弗里克等人,2022)。与现已灭绝的巨型食果动物的这种历史相互作用也可能在当前的物种和种群中留下了印记。例如,马达加斯加棕榈树物种在组合中的更替(即β多样性)至少部分是由历史上与现已灭绝的大型食果动物(主要在该岛西部地区)的共存所决定的(Méndez、Viana等,2017)。 ,2022a)。同样,棕榈果大小的新热带分布最好通过考虑过去(现已灭绝)的巨型食果动物来解释(Lim 等人,2020)。然而,历史上与现已灭绝的巨型食果动物的相互作用是否也影响了植物种群遗传学仍然很大程度上未知。
在马达加斯加,巨型动物群的减少始于大约 1000 年前,主要是由于人类影响的增加(Crowley,2010)。狩猎以及向畜牧和农耕的过渡,改变了地貌和巨型动物的栖息地,最终导致所有巨型动物的灭绝(Godfrey 等,2019;Li 等,2020)。其中一些已灭绝的巨型动物物种,例如巨型狐猴(例如 Pachylemur spp. 和 Archaeolemur spp.)、象鸟(例如 Aepyornis spp. 和 Vorombe spp.)和巨型陆龟(Aldabrachelys spp.),可能是食果者(Godfrey)等人,2004 年;佩德罗诺等人,2013 年)。有趣的是,花粉化石表明,马达加斯加特有巨型动物的灭绝与依赖巨型食果动物传播种子的树木数量逐渐减少同时发生(Domic et al., 2021)。然而,没有直接证据表明任何植物物种的灭绝是由于其巨型食果动物相互作用伙伴的灭绝而发生的。相反,有人认为,由于人类驯化,一些适应大型食果动物种子传播的植物物种在当今的生态系统中得以持续存在(Kistler等人,2015),以及体型较小的食果动物的二次种子传播(Blanco等人,2015)。 ,2019),或其他非生物形式的传播(例如通过河流;Guimarães 等人,2008)。
环境和景观相关因素可能通过非果食相关的扩散过程塑造了植物种群遗传学(Jiang 等,2019;Sexton 等,2014;Siepielski 等,2017)。环境适宜性(即特定地理区域为物种生存和繁衍提供适当条件的程度)可以充当扩散的障碍或走廊(McRae,2006;Wang&Bradburd,2014),从而影响遗传植物种群的分化。例如,马达加斯加的河流充当了传播的障碍,导致无叶香草兰花(香草属;Andriamihaja 等人,2021)具有较高的遗传结构,而它们则充当了巨型果树 Eligmocarpus cynometroides(豆科)的传播走廊。 ,使其能够在多个生物群落中传播(Devey et al., 2013)。此外,森林覆盖,特别是河岸森林,在连接森林斑块和构建马达加斯加特有树木的遗传多样性方面发挥着重要作用(即Noronhia spinifolia,Salmona等,2022)。
人类活动也是当今植物种群遗传学的主要决定因素(Smith 等,2020)。例如,人造基础设施(例如定居点或道路网络)对生态系统的破碎可能会限制大型脊椎动物(例如食果哺乳动物)在景观中的移动,从而导致具有大型脊椎动物分散果实的植物的传播限制(Tucker等人)等,2021)。然而,通过移动植物种子,人类也可能促进长距离传播事件(Wichmann et al., 2008),从而可能减少植物种群之间的遗传分化(Arredondo et al., 2018;Bullock et al., 2018)。此外,未铺砌的道路可以作为植物的种子走廊,因为一些陆生哺乳动物选择它们作为粪便标记,促进种子沿路边传播(Suárez-Esteban 等,2013)。因此,人类活动可能会增加植物种群之间的基因流动,也可能会破坏基因流动,具体取决于具体情况。
在这里,我们评估了马达加斯加过去的巨型食果动物提供的传播服务所产生的模式是否仍然可以在当前棕榈(槟榔科)种群的基因组中检测到。我们重点关注棕榈树,因为有几个物种被认为是马达加斯加生态系统中的“不合时宜”,因为它们的巨型动物水果尺寸(长度>4厘米,以下称为巨型水果)似乎不适应当前食果动物池的传播(Albert-Daviaud)等人,2020)。我们选择了该岛西部的物种,那里过去可能是巨型食果动物最丰富的地方(Crowley 等,2011)。这些稀树草原脊椎动物分散的棕榈树种可分为三个果实大小类别:大型巨型水果(平均长度为 30 厘米 - Borassus madagascariensis)、中型巨型水果(5.5 厘米 - Hyphaene coriacea、4.4 厘米 - Bismarckia nobilis)和小型水果( <4 厘米;1.3 厘米——Chrysalidocarpus madagascariensis [以前称为 Dypsis madagascariensis,Eiserhardt 等,2022]; C. madagascariensis 相对较小的果实仍然可以被现存的食果动物传播(例如 Eulemur macaco;Adany 等人,1994),而其他三个物种的巨型果实太大,无法被任何现存的本地食果动物吞食和传播。马达加斯加(Perry & Hartstone-Rose,2010)。此外,结有中等大小巨型水果(H. coriacea 和 B. nobilis)的棕榈树被人类广泛用于房屋建筑、篮筐或食品(Rakotoarinivo 等人,2020)。因此,人类介导的扩散可能有助于这些物种在过去 1000 年的持续存在和遗传。最后,这三种巨型水果属于 Borasseae 族(B. madagascariensis、H. coriacea 和 B. nobilis) 是雌雄异株的,雄性和雌性个体分开,而 C. madagascariensis 是雌雄同株(雄性和雌性花在同一个体上)。人们对这些棕榈树的传粉者知之甚少,但花朵相对较小且不显眼,表明昆虫传粉者的范围很广(Henderson,1986)。
表 1. 平均果实长度(厘米)、抽样群体数量以及平均(±标准差,SD)群体水平历史遗传分化(Fst)以及近期群体间(M-among-pops)和群体内(M-within-pops)四种棕榈树的种群迁移率。有关更详细的信息和按群体计算的值,请参阅支持信息中的表 S5 和 S6。
| 果长(厘米) | 人口数量 | 平均 Fst (SD) | 流行音乐中的平均 M (SD) | 平均 M 内弹出 (SD) | |
|---|---|---|---|---|---|
|
马达加斯加白鲸 |
30 | 3 | 0.192 (0.035) | 0.031 (0.007) | 0.938 (0.015) |
| 珊瑚菌 | 5.5 | 7 | 0.129 (0.034) | 0.025 (0.001) | 0.847 (0.008) |
| 俾斯麦 | 4.4 | 8 | 0.125 (0.039) | 0.029 (0.019) | 0.798 (0.047) |
|
马达加斯加金凤花 |
1.3 | 7 | 0.188 (0.061) | 0.025 (0.004) | 0.847 (0.015) |
我们假设(H1),历史上巨型果树食动物的长距离扩散事件促进了种群之间的基因流动,从而在巨型果棕榈种群当前的遗传学上留下了印记。具体来说,我们预计历史上共享更多巨型食果动物物种的棕榈种群通过频繁的大型食果动物介导的种子传播和基因流而更加紧密地联系在一起,从而导致比共享较少巨型食果动物物种的种群具有更大的遗传相似性(即较低的遗传分化)。相比之下,小果棕榈种群的遗传分化预计主要是由现存体型较小的食果动物过去和正在进行的扩散所决定的,现存食果动物的共享数量较多,导致种群间遗传分化较低。此外,我们假设森林、河流和环境适宜性(H2)和人类(H3)等景观相关特征促进了棕榈种群的遗传分化和近期迁徙率,可能是通过取代巨型食果动物灭绝后的种子传播服务来实现的。具体来说,我们预计森林覆盖、环境适宜性和河流密度已成为基因流动的走廊,从而减少遗传分化并提高种群之间的迁移率。同样,人口密度和道路密度可能通过促进植物种群之间的扩散(直接通过植物材料的移动和/或间接通过为沿道路边缘移动的现有食果动物提供走廊)来增加人口连通性。 另外,人类和道路可能会造成景观碎片化和人口连通性降低,从而增加人口分化并降低人口之间的迁移率。
为了检验这些假设,我们将棕榈种群水平的基因组数据与过去对大型食果动物分布、当前食果动物分布、景观和人类影响变量的推论进行了整合。我们应用线性混合效应模型来理清棕榈种群遗传分化和近期迁徙率的果食相关、景观相关和人类相关驱动因素。我们的研究结果为了解过去与巨型食果动物的相互作用如何影响马达加斯加植物的遗传学提供了新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号