当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The interaction of thiocyanate with peptides—A computational study
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-05-25 , DOI: 10.1002/jcc.27440 Orlando Crescenzi 1 , Giuseppe Graziano 2
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-05-25 , DOI: 10.1002/jcc.27440 Orlando Crescenzi 1 , Giuseppe Graziano 2
Affiliation
|
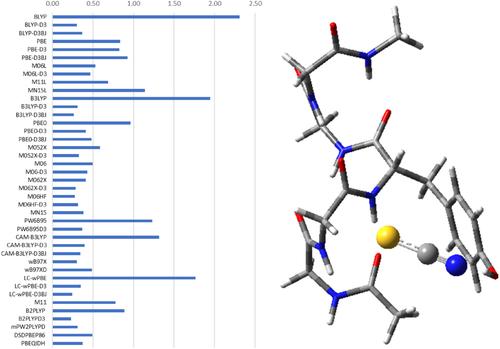
According to the Hofmeister series, thiocyanate is the strongest “salting in” anion. In fact, it has a strong denaturant activity against the native state of globular proteins. A molecular level rationalization of the Hofmeister series is still missing, and therefore the denaturant activity of thiocyanate also awaits a robust explanation. In the last years, different types of experimental studies have shown that thiocyanate is capable to directly interact with both polar and nonpolar groups of polypeptide chains. This finding has been scrutinized via a careful computational procedure based on density functional theory approaches. The results indicate that thiocyanate is able to make H‐bonds via both the nitrogen and sulfur atom, and to make strong van der Waals interactions with almost all the groups of polypeptide chains, regardless of their polarity.
中文翻译:

硫氰酸盐与肽的相互作用——计算研究
根据霍夫迈斯特系列,硫氰酸根是最强的“盐化”阴离子。事实上,它对球状蛋白的天然状态具有很强的变性活性。霍夫迈斯特级数的分子水平合理化仍然缺失,因此硫氰酸盐的变性活性也有待强有力的解释。近年来,不同类型的实验研究表明硫氰酸盐能够直接与多肽链的极性和非极性基团相互作用。这一发现已经通过基于密度泛函理论方法的仔细计算程序进行了仔细审查。结果表明,硫氰酸盐能够通过氮原子和硫原子形成氢键,并与几乎所有多肽链基团产生强烈的范德华相互作用,无论其极性如何。
更新日期:2024-05-25
中文翻译:

硫氰酸盐与肽的相互作用——计算研究
根据霍夫迈斯特系列,硫氰酸根是最强的“盐化”阴离子。事实上,它对球状蛋白的天然状态具有很强的变性活性。霍夫迈斯特级数的分子水平合理化仍然缺失,因此硫氰酸盐的变性活性也有待强有力的解释。近年来,不同类型的实验研究表明硫氰酸盐能够直接与多肽链的极性和非极性基团相互作用。这一发现已经通过基于密度泛函理论方法的仔细计算程序进行了仔细审查。结果表明,硫氰酸盐能够通过氮原子和硫原子形成氢键,并与几乎所有多肽链基团产生强烈的范德华相互作用,无论其极性如何。












































 京公网安备 11010802027423号
京公网安备 11010802027423号