当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulating Zn2+ Solvation Shell Through Charge-Concentrated Anions for High Zn Plating/Stripping Coulombic Efficiency
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-05-25 , DOI: 10.1002/adfm.202405145
Diantao Li 1 , Tianjiang Sun 1 , Tao Ma 1 , Weijia Zhang 1 , Qiong Sun 1 , Min Cheng 1 , Zhengtai Zha 1 , Weiwei Xie 1 , Zhanliang Tao 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2024-05-25 , DOI: 10.1002/adfm.202405145
Diantao Li 1 , Tianjiang Sun 1 , Tao Ma 1 , Weijia Zhang 1 , Qiong Sun 1 , Min Cheng 1 , Zhengtai Zha 1 , Weiwei Xie 1 , Zhanliang Tao 1
Affiliation
|
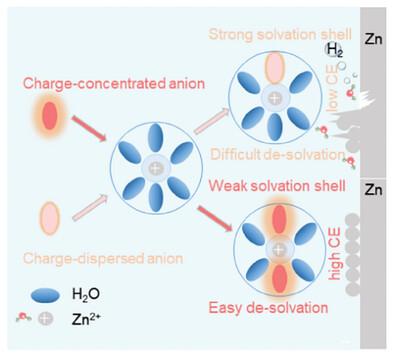
The plating/stripping efficiency of zinc (Zn) is directly related to the efficiency of zinc utilization and cycle stability of the battery, which is affected by factors such as the solvated water-related hydrogen evolution reaction (HER), Zn corrosion, and dendrite formation. Therefore, creating a weak solvate shell for Zn2+ with reduced solvated water molecules can promote stable deposition and stripping of the zinc anode. In this work, a novel approach using the concentrated charge effect of anions is proposed to remove the solvated water and improve the efficiency of Zn plating/stripping. 3 mol kg−1 (3 m) ZnCl2, Zn(ClO4)2, and Zn(BF4)2 electrolytes are used as the representatives to investigate how anions regulate the solvent shell of zinc ion to achieve high Zn plating/stripping Coulombic efficiency (CE). Computational results show that Cl− has a more concentrated charge compared to ClO4− and BF4−, indicating a stronger interaction with Zn2+. This concentrated charge effect reduces the number of water molecules in Zn2+ solvation structures. Benefiting from weak solvent structure, the average coulomb efficiency, and cycling stability of the Zn─Cu asymmetric cell using ZnCl2 electrolyte is better. Additionally, the Zn-NaV3O8 full cell of the ZnCl2 electrolyte exhibits good electrochemical performance.
中文翻译:

通过电荷浓阴离子调节 Zn2+ 溶剂化壳,以实现高 Zn 电镀/剥离库仑效率
锌 (Zn) 的电镀/剥离效率与电池的锌利用效率和循环稳定性直接相关,受溶剂化水相关析氢反应 (HER)、Zn 腐蚀和枝晶形成等因素的影响。因此,用还原的溶剂化水分子为 Zn2+ 创建一个弱溶剂化壳层可以促进锌阳极的稳定沉积和剥离。在这项工作中,提出了一种利用阴离子的浓电荷效应的新方法来去除溶剂化水并提高镀锌/剥离的效率。以 3 mol kg−1 (3 m) ZnCl2、Zn(ClO4)2 和 Zn(BF4)2 电解质为代表,研究阴离子如何调节锌离子的溶剂壳以实现高 Zn 镀锌/剥离库仑效率 (CE)。计算结果表明,与 ClO4− 和 BF4− 相比,Cl− 具有更浓的电荷,表明与 Zn2+ 的相互作用更强。这种集中电荷效应减少了 Zn2+ 溶剂化结构中水分子的数量。得益于弱溶剂结构,使用 ZnCl2 电解质的 Zn─Cu 不对称电池的平均库仑效率和循环稳定性更好。此外,ZnCl2 电解质的 Zn-NaV3O8 全电池表现出良好的电化学性能。
更新日期:2024-05-25
中文翻译:

通过电荷浓阴离子调节 Zn2+ 溶剂化壳,以实现高 Zn 电镀/剥离库仑效率
锌 (Zn) 的电镀/剥离效率与电池的锌利用效率和循环稳定性直接相关,受溶剂化水相关析氢反应 (HER)、Zn 腐蚀和枝晶形成等因素的影响。因此,用还原的溶剂化水分子为 Zn2+ 创建一个弱溶剂化壳层可以促进锌阳极的稳定沉积和剥离。在这项工作中,提出了一种利用阴离子的浓电荷效应的新方法来去除溶剂化水并提高镀锌/剥离的效率。以 3 mol kg−1 (3 m) ZnCl2、Zn(ClO4)2 和 Zn(BF4)2 电解质为代表,研究阴离子如何调节锌离子的溶剂壳以实现高 Zn 镀锌/剥离库仑效率 (CE)。计算结果表明,与 ClO4− 和 BF4− 相比,Cl− 具有更浓的电荷,表明与 Zn2+ 的相互作用更强。这种集中电荷效应减少了 Zn2+ 溶剂化结构中水分子的数量。得益于弱溶剂结构,使用 ZnCl2 电解质的 Zn─Cu 不对称电池的平均库仑效率和循环稳定性更好。此外,ZnCl2 电解质的 Zn-NaV3O8 全电池表现出良好的电化学性能。

































 京公网安备 11010802027423号
京公网安备 11010802027423号