当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trifluoromethoxylation of Arynes Using 2,4-Dinitro-1-(trifluoromethoxybenzene) as Trifluoromethoxide Anion Source
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2024-05-24 , DOI: 10.1002/ejoc.202400388 Lilian Wisson 1 , Gilles Hanquet 1 , Fabien Toulgoat 2 , Thierry Billard 3 , Armen Panossian 1 , Frederic R. Leroux 4
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2024-05-24 , DOI: 10.1002/ejoc.202400388 Lilian Wisson 1 , Gilles Hanquet 1 , Fabien Toulgoat 2 , Thierry Billard 3 , Armen Panossian 1 , Frederic R. Leroux 4
Affiliation
|
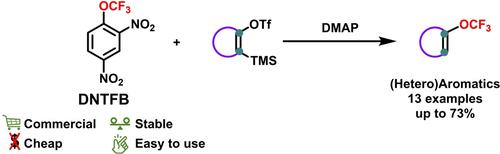
The trifluoromethoxylation of arynes has been little reported in the literature mainly due to the limited number of stable trifluoromethoxide anion sources existing. We describe herein the trifluoromethoxylation of arynes using 2,4-dinitro-1-(trifluoromethoxy)benzene (DNTFB) as a cheap and easy-to-use source of F3CO−. The released trifluoromethoxide anion was used as both a source of fluoride to generate the aryne and a nucleophile for the trifluoromethoxylation reaction.
中文翻译:

使用 2,4-二硝基-1-(三氟甲氧基苯) 作为三氟甲氧基阴离子源进行芳烃的三氟甲氧基化
芳烃的三氟甲氧基化在文献中报道很少,这主要是由于现有的稳定的三氟甲氧基阴离子源的数量有限。我们在此描述了使用 2,4-二硝基-1-(三氟甲氧基)苯 (DNTFB) 作为廉价且易于使用的 F 3 CO − 来源的芳烃的三氟甲氧基化。释放的三氟甲氧基阴离子既用作产生芳基的氟化物源,又用作三氟甲氧基化反应的亲核试剂。
更新日期:2024-05-24
中文翻译:

使用 2,4-二硝基-1-(三氟甲氧基苯) 作为三氟甲氧基阴离子源进行芳烃的三氟甲氧基化
芳烃的三氟甲氧基化在文献中报道很少,这主要是由于现有的稳定的三氟甲氧基阴离子源的数量有限。我们在此描述了使用 2,4-二硝基-1-(三氟甲氧基)苯 (DNTFB) 作为廉价且易于使用的 F 3 CO − 来源的芳烃的三氟甲氧基化。释放的三氟甲氧基阴离子既用作产生芳基的氟化物源,又用作三氟甲氧基化反应的亲核试剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号