当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Understanding of Anthracene Hydrocracking over HY Zeolite Encapsulated Single-Atom Pt Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-05-23 , DOI: 10.1021/acscatal.4c01706 Wenru Zhao 1 , Hui Yu 1 , Shaozhong Peng 2 , Wei Liu 2 , Weiwei Zhang 3 , Donghai Mei 1, 4
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-05-23 , DOI: 10.1021/acscatal.4c01706 Wenru Zhao 1 , Hui Yu 1 , Shaozhong Peng 2 , Wei Liu 2 , Weiwei Zhang 3 , Donghai Mei 1, 4
Affiliation
|
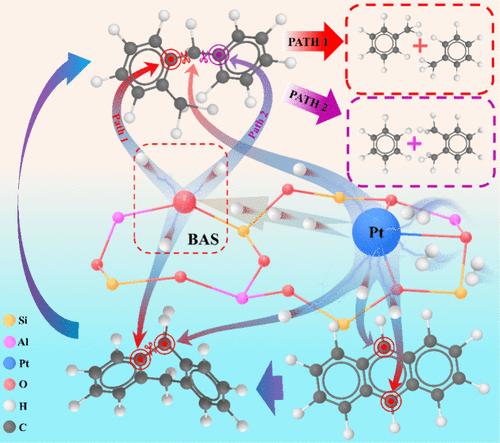
The hydrocracking of polycyclic aromatic hydrocarbons (PAHs) leading to the production of benzene, toluene, and xylene (BTX) is one of most important industrial petrochemical processes. Although experiencing extensive experimental effort and industrial practice, the underlying hydrocracking reaction mechanisms of PAHs over zeolite-supported metal catalysts are still elusive. In particular, when, where, and how the aromatic rings of PAHs are open via C–C bond breaking is not clear. In the present work, the hydrocracking reaction pathways of anthracene over the HY zeolite encapsulated single-atom Pt catalyst (Pt1/HY) as a demonstration case for the hydrocracking of PAHs have been investigated using density functional theory (DFT) calculations. The ring-opening processes of the terminal and the central rings of anthracene with respect to the saturated hydrogenation degrees of aromatic rings and the hydrogen sources, which result in different BTX products, have been systematically examined. The hydride transfer over the same aromatic ring is facile, while it is kinetically hindered at the connecting C atoms (Ca) between the neighboring aromatic rings. The hydride transfer between the terminal and the central rings, in the case of the isomerization of 2,3-dihydroanthracene to 9,10-dihydroanthracene, can be achieved through the intermolecular hydride transfer mechanism with the assistance of anthracene. Compared to the hydrogenation from the Pt1 site, the addition of a proton from the Brønsted acidic sites (BAS) on the aromatic ring of partially hydrogenated anthracene would pronouncedly weaken the C–C bond, resulting in the central ring-opening process. DFT calculation results show the central ring opening is kinetically favorable in the anthracene hydrocracking over the Pt1/HY catalyst, generating BTX as the major product rather than butylbenzene and n-butane. The first protonation step by BAS on both rings is the most kinetically relevant step. In addition, the hydrocracking reaction of branched PAHs, using octylanthracene as the probe molecule, has also been investigated. It has been found that the dealkylation of octylanthracene at the ring-branched chain connection position is kinetically more feasible than the central ring opening and the cleavage of the octyl chain. The present work provides a comprehensive and insightful mechanistic understanding of the hydrocracking reaction pathways of PAHs over bifunctional HY zeolite supported metal catalysts.
中文翻译:

HY 沸石封装单原子 Pt 催化剂上蒽加氢裂化的机理理解
多环芳烃 (PAH) 的加氢裂化生产苯、甲苯和二甲苯 (BTX),是最重要的工业石化过程之一。尽管经历了广泛的实验努力和工业实践,多环芳烃在沸石负载金属催化剂上的基本加氢裂化反应机制仍然难以捉摸。特别是,多环芳烃的芳环何时、何地以及如何通过 C-C 键断裂打开尚不清楚。在目前的工作中,作为多环芳烃加氢裂化的示范案例,使用密度泛函理论研究了 HY 沸石封装的单原子 Pt 催化剂(Pt 1 /HY)上的蒽加氢裂化反应路径( DFT)计算。系统地研究了蒽末端和中心环的开环过程与芳环饱和氢化度和氢源的关系,从而产生不同的BTX产物。同一芳环上的氢化物转移很容易,但在相邻芳环之间的连接碳原子(C a )处受到动力学阻碍。 2,3-二氢蒽异构化为9,10-二氢蒽时,端环与中心环之间的氢化物转移可以在蒽的辅助下通过分子间氢化物转移机制实现。与 Pt 1 位点的氢化相比,部分氢化蒽芳环上的 Brønsted 酸性位点(BAS)添加一个质子会明显削弱 C-C 键,导致中心开环过程。 DFT计算结果表明,在Pt 1 /HY催化剂上,中心开环在动力学上有利于蒽加氢裂化,生成BTX作为主要产物,而不是丁苯和正丁烷。 BAS 在两个环上的第一个质子化步骤是动力学上最相关的步骤。此外,还研究了以辛基蒽为探针分子的支链多环芳烃的加氢裂化反应。研究发现,辛基蒽在环支链连接位置的脱烷基化比中心开环和辛基链断裂在动力学上更可行。目前的工作为双功能 HY 沸石负载金属催化剂上 PAH 的加氢裂化反应途径提供了全面而深刻的机理理解。
更新日期:2024-05-23
中文翻译:

HY 沸石封装单原子 Pt 催化剂上蒽加氢裂化的机理理解
多环芳烃 (PAH) 的加氢裂化生产苯、甲苯和二甲苯 (BTX),是最重要的工业石化过程之一。尽管经历了广泛的实验努力和工业实践,多环芳烃在沸石负载金属催化剂上的基本加氢裂化反应机制仍然难以捉摸。特别是,多环芳烃的芳环何时、何地以及如何通过 C-C 键断裂打开尚不清楚。在目前的工作中,作为多环芳烃加氢裂化的示范案例,使用密度泛函理论研究了 HY 沸石封装的单原子 Pt 催化剂(Pt 1 /HY)上的蒽加氢裂化反应路径( DFT)计算。系统地研究了蒽末端和中心环的开环过程与芳环饱和氢化度和氢源的关系,从而产生不同的BTX产物。同一芳环上的氢化物转移很容易,但在相邻芳环之间的连接碳原子(C a )处受到动力学阻碍。 2,3-二氢蒽异构化为9,10-二氢蒽时,端环与中心环之间的氢化物转移可以在蒽的辅助下通过分子间氢化物转移机制实现。与 Pt 1 位点的氢化相比,部分氢化蒽芳环上的 Brønsted 酸性位点(BAS)添加一个质子会明显削弱 C-C 键,导致中心开环过程。 DFT计算结果表明,在Pt 1 /HY催化剂上,中心开环在动力学上有利于蒽加氢裂化,生成BTX作为主要产物,而不是丁苯和正丁烷。 BAS 在两个环上的第一个质子化步骤是动力学上最相关的步骤。此外,还研究了以辛基蒽为探针分子的支链多环芳烃的加氢裂化反应。研究发现,辛基蒽在环支链连接位置的脱烷基化比中心开环和辛基链断裂在动力学上更可行。目前的工作为双功能 HY 沸石负载金属催化剂上 PAH 的加氢裂化反应途径提供了全面而深刻的机理理解。































 京公网安备 11010802027423号
京公网安备 11010802027423号