当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Novel Pyridazine Herbicides Targeting Phytoene Desaturase with Scaffold Hopping
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-05-23 , DOI: 10.1021/acs.jafc.3c09350 Chao Chen 1, 2 , Qiong Lei 1 , Wang Geng 1 , Daoping Wang 2 , Xiuhai Gan 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-05-23 , DOI: 10.1021/acs.jafc.3c09350 Chao Chen 1, 2 , Qiong Lei 1 , Wang Geng 1 , Daoping Wang 2 , Xiuhai Gan 1
Affiliation
|
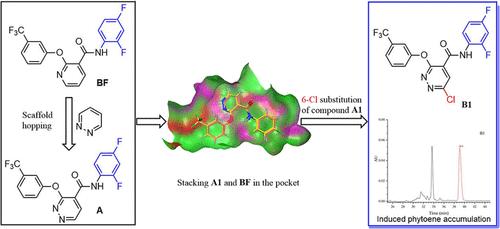
Phytoene desaturase (PDS) is a critical functional enzyme in blocking ζ-carotene biosynthesis and is one of the bleaching herbicide targets. At present, norflurazon (NRF) is the only commercial pyridazine herbicide targeting PDS. Therefore, developing new and diverse pyridazine herbicides targeting PDS is urgently required. In this study, diflufenican (BF) was used as the lead compound, and a scaffold-hopping strategy was employed to design and synthesize some pyridazine derivatives based on the action mode of BF and PDS. The preemergence herbicidal activity tests revealed that compound 6-chloro-N-(2,4-difluorophenyl)-3-(3-(trifluoromethyl)phenoxy)pyridazine-4-carboxamide (B1) with 2,4-diF substitution in the benzeneamino ring showed 100% inhibition rates against the roots and stems of Echinochloa crus-galli and Portulaca oleracea at 100 μg/mL, superior to the inhibition rates of BF. Meanwhile, compound B1 demonstrated excellent postemergence herbicidal activity against broadleaf weeds, which was similar to that of BF (inhibition rate of 100%) but superior to that of NRF. This indicated that 6-Cl in the pyridazine ring is the key group for postemergence herbicidal activity. In addition, compound B1 could induce downregulation of PDS gene expression, 15-cis-phytoene accumulation, and Y(II) deficiency and prevent photosynthesis. Therefore, B1 can be considered as a promising candidate for developing high-efficiency PDS inhibitors.
中文翻译:

通过支架跳跃发现针对八氢番茄红素去饱和酶的新型哒嗪除草剂
八氢番茄红素去饱和酶(PDS)是阻断 β-胡萝卜素生物合成的关键功能酶,也是漂白除草剂的靶标之一。目前,诺氟草酮( NRF )是唯一针对PDS的商品化哒嗪类除草剂。因此,迫切需要开发针对PDS的新型、多样化的哒嗪类除草剂。本研究以氟苯尼康( BF )为先导化合物,基于BF和PDS的作用模式,采用支架跳跃策略设计合成了一些哒嗪衍生物。芽前除草活性测试表明化合物6-氯-N- (2,4-二氟苯基)-3-(3-(三氟甲基)苯氧基)哒嗪-4-甲酰胺( B1 )苯氨基上有2,4-二F取代环在100 μg/mL时对稗草和马齿苋的根和茎的抑制率为100%,优于BF的抑制率。同时,化合物B1对阔叶杂草表现出优异的芽后除草活性,与BF相似(抑制率100%),但优于NRF 。这表明哒嗪环中的6-Cl是芽后除草活性的关键基团。此外,化合物B1可诱导PDS基因表达下调、15-顺式-八氢番茄红素积累和Y(II)缺乏并阻止光合作用。因此, B1可以被认为是开发高效PDS抑制剂的有希望的候选者。
更新日期:2024-05-23
中文翻译:

通过支架跳跃发现针对八氢番茄红素去饱和酶的新型哒嗪除草剂
八氢番茄红素去饱和酶(PDS)是阻断 β-胡萝卜素生物合成的关键功能酶,也是漂白除草剂的靶标之一。目前,诺氟草酮( NRF )是唯一针对PDS的商品化哒嗪类除草剂。因此,迫切需要开发针对PDS的新型、多样化的哒嗪类除草剂。本研究以氟苯尼康( BF )为先导化合物,基于BF和PDS的作用模式,采用支架跳跃策略设计合成了一些哒嗪衍生物。芽前除草活性测试表明化合物6-氯-N- (2,4-二氟苯基)-3-(3-(三氟甲基)苯氧基)哒嗪-4-甲酰胺( B1 )苯氨基上有2,4-二F取代环在100 μg/mL时对稗草和马齿苋的根和茎的抑制率为100%,优于BF的抑制率。同时,化合物B1对阔叶杂草表现出优异的芽后除草活性,与BF相似(抑制率100%),但优于NRF 。这表明哒嗪环中的6-Cl是芽后除草活性的关键基团。此外,化合物B1可诱导PDS基因表达下调、15-顺式-八氢番茄红素积累和Y(II)缺乏并阻止光合作用。因此, B1可以被认为是开发高效PDS抑制剂的有希望的候选者。































 京公网安备 11010802027423号
京公网安备 11010802027423号