Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative and Extractive Desulfurization of Fuel Oils Catalyzed by N-Carboxymethyl Pyridinium Acetate and N-Carboxyethyl Pyridinium Acetate Acidic Ionic Liquids: Experimental and Computational DFT Study
ACS Omega ( IF 3.7 ) Pub Date : 2024-05-21 , DOI: 10.1021/acsomega.3c09975 Amani Sager 1 , Shofiur Rahman 2 , Syed A Imtiaz 1 , Yan Zhang 1 , Abdullah Alodhayb 2 , Paris E Georghiou 3 , Mahmoud Al-Gawati 2
ACS Omega ( IF 3.7 ) Pub Date : 2024-05-21 , DOI: 10.1021/acsomega.3c09975 Amani Sager 1 , Shofiur Rahman 2 , Syed A Imtiaz 1 , Yan Zhang 1 , Abdullah Alodhayb 2 , Paris E Georghiou 3 , Mahmoud Al-Gawati 2
Affiliation
|
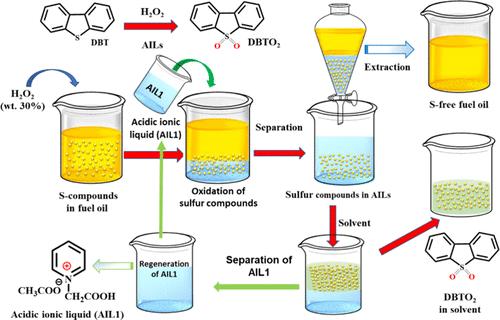
This study reports on the synthesis, characterization, and application of two acidic ionic liquids, namely, N-carboxymethylpyridinium acetate ([HO2CCH2Py][CH3CO2] or AIL1) and N-carboxyethylpyridinium acetate ([HO2C(CH2)2Py][CH3CO2] or AIL2), as both extractants and catalysts for the oxidative and extractive desulfurization (OEDS) of model fuel oils containing heteroaromatic sulfur compounds. The structural properties of the synthesized acidic ionic liquids (ILs) were confirmed by 1H NMR, 13C NMR, and FT-IR spectroscopic analysis. To optimize the performance of the acidic AILs in the desulfurization process, the effects of different parameters, such as H2O2 dosage, reaction time, and temperatures, were investigated. The experimental results showed that AIL1 has exceptionally high desulfurization–extraction rates, with values of 99.8%, 97.8%, and 95.4%, for DBT, BT, and 4,6-DMDBT, respectively, under the optimum conditions established. Under the same conditions, the desulfurization-extraction rates using AIL2 reached 91.6%, 87.3%, and 82.4%, respectively, for DBT, 4, 6-DMDBT, and BT. Both ionic liquids can be recycled up to 9 times without a significant decrease in their sulfur removal efficiencies. Furthermore, density functional theory (DFT) calculations were conducted to evaluate the electronic interaction energies (ΔIE) between the AILs with each of the sulfur-containing compounds and their putative oxidized products. The computational findings strongly supported the experimental outcomes.
中文翻译:

N-羧甲基吡啶乙酸盐和 N-羧乙基吡啶乙酸盐酸性离子液体催化燃料油的氧化和萃取脱硫:实验和计算 DFT 研究
本研究报告了两种酸性离子液体的合成、表征和应用,即N-羧甲基吡啶乙酸盐([HO 2 CCH 2 Py][CH 3 CO 2 ]或AIL1 )和N-羧乙基吡啶乙酸盐([HO 2 C (CH 2 ) 2 Py][CH 3 CO 2 ]或AIL2 ),作为含有杂芳族硫化合物的模型燃料油的氧化和萃取脱硫(OEDS)的萃取剂和催化剂。通过1 H NMR、 13 C NMR 和 FT-IR 光谱分析证实了合成的酸性离子液体(IL)的结构性质。为了优化脱硫过程中酸性 AIL 的性能,研究了不同参数(例如 H 2 O 2剂量、反应时间和温度)的影响。实验结果表明,在建立的最佳条件下, AIL1对DBT、BT和4,6-DMDBT具有极高的脱硫萃取率,其值分别为99.8%、97.8%和95.4%。在相同条件下, AIL2对DBT、4、6-DMDBT和BT的脱硫萃取率分别达到91.6%、87.3%和82.4%。两种离子液体均可循环利用多达 9 次,而除硫效率不会显着降低。此外,还进行了密度泛函理论 (DFT) 计算,以评估 AIL 与每种含硫化合物及其假定氧化产物之间的电子相互作用能 (ΔIE)。 计算结果有力地支持了实验结果。
更新日期:2024-05-21
中文翻译:

N-羧甲基吡啶乙酸盐和 N-羧乙基吡啶乙酸盐酸性离子液体催化燃料油的氧化和萃取脱硫:实验和计算 DFT 研究
本研究报告了两种酸性离子液体的合成、表征和应用,即N-羧甲基吡啶乙酸盐([HO 2 CCH 2 Py][CH 3 CO 2 ]或AIL1 )和N-羧乙基吡啶乙酸盐([HO 2 C (CH 2 ) 2 Py][CH 3 CO 2 ]或AIL2 ),作为含有杂芳族硫化合物的模型燃料油的氧化和萃取脱硫(OEDS)的萃取剂和催化剂。通过1 H NMR、 13 C NMR 和 FT-IR 光谱分析证实了合成的酸性离子液体(IL)的结构性质。为了优化脱硫过程中酸性 AIL 的性能,研究了不同参数(例如 H 2 O 2剂量、反应时间和温度)的影响。实验结果表明,在建立的最佳条件下, AIL1对DBT、BT和4,6-DMDBT具有极高的脱硫萃取率,其值分别为99.8%、97.8%和95.4%。在相同条件下, AIL2对DBT、4、6-DMDBT和BT的脱硫萃取率分别达到91.6%、87.3%和82.4%。两种离子液体均可循环利用多达 9 次,而除硫效率不会显着降低。此外,还进行了密度泛函理论 (DFT) 计算,以评估 AIL 与每种含硫化合物及其假定氧化产物之间的电子相互作用能 (ΔIE)。 计算结果有力地支持了实验结果。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号