当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Compatibility Study between 50% H2O2/H2SO4 Solution and Some Common Organic Solvents
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-05-24 , DOI: 10.1021/acs.oprd.3c00468 Shichun Weng 1 , Juan Zhou 2 , Yingtao Tian 3 , Xigui Jiang 3 , Rong Chen 1 , Jinyao Hu 1 , Zichao Guo 1 , Liping Chen 1 , Wanghua Chen 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-05-24 , DOI: 10.1021/acs.oprd.3c00468 Shichun Weng 1 , Juan Zhou 2 , Yingtao Tian 3 , Xigui Jiang 3 , Rong Chen 1 , Jinyao Hu 1 , Zichao Guo 1 , Liping Chen 1 , Wanghua Chen 1
Affiliation
|
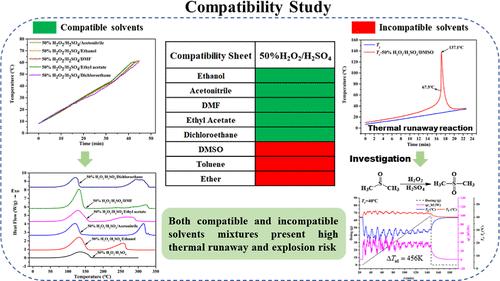
In this article, a mixture of sulfuric acid and 50% hydrogen peroxide solution was used to produce Caro’s acid, which serves as an oxidant frequently encountered in the fine chemical industry. The chemical compatibility between 50% H2O2/H2SO4 and organic solvents is an important safety issue. The compatibility of 50% H2O2/H2SO4 and several organic solvents, including ethanol, acetonitrile, ethyl acetate, DMF, dichloroethane, DMSO, ether, and toluene, was studied by reaction calorimetry and thermal analysis techniques. It was found that the first five solvents presented good compatibility with 50% H2O2/H2SO4. DSC tests indicated that these five solvents almost did not react with 50% H2O2/H2SO4 before the onset decomposition temperature of 50% H2O2/H2SO4 (∼93.3 °C). RADEX tests showed that the reactions of 50% H2O2/H2SO4 with these solvents released heats from 800 to 1100 J g–1, and the pressure effect was obvious for these mixtures. In contrast, the last three solvents, namely, DMSO, ether, and toluene, were incompatible with 50% H2O2/H2SO4, exhibiting a significant exothermic signal at lower temperatures. Most interestingly, the mixture of DMSO and 50% H2O2/H2SO4 presented a thermal runaway phenomenon below 40 °C. The causes of this thermal runaway incident were confirmed to be the oxidation of DMSO by Caro’s acid. Reaction calorimetry tests also indicated that the oxidation rate of DMSO was fast even at 40 °C, and the adiabatic temperature rise for the oxidation of DMSO was higher than 400 K.
中文翻译:

50% H2O2/H2SO4 溶液与一些常见有机溶剂的相容性研究
在本文中,使用硫酸和50%过氧化氢溶液的混合物来生产卡罗酸,它是精细化工行业中常见的氧化剂。 50% H 2 O 2 /H 2 SO 4 与有机溶剂之间的化学相容性是一个重要的安全问题。 50% H 2 O 2 /H 2 SO 4 与多种有机溶剂(包括乙醇、乙腈、乙酸乙酯)的相容性、DMF、二氯乙烷、DMSO、乙醚和甲苯,通过反应量热法和热分析技术进行了研究。结果发现,前5种溶剂与50% H 2 O 2 /H 2 SO 4 表现出良好的相容性。 DSC测试表明,这五种溶剂在发生前几乎不与50% H 2 O 2 /H 2 SO 4 发生反应50% H 2 O 2 /H 2 SO 4 的分解温度 (∼93.3 °C)。 RADEX 测试表明,50% H 2 O 2 /H 2 SO 4 与这些溶剂的反应释放热量从 800 到1100 J g –1 ,压力对这些混合物的影响很明显。相比之下,后三种溶剂,即 DMSO、乙醚和甲苯,与 50% H 2 O 2 /H 2 SO 4 ,在较低温度下表现出显着的放热信号。最有趣的是,DMSO和50% H 2 O 2 /H 2 SO 4 的混合物在低于40℃时出现热失控现象°C。此次热失控事件的原因被确认为卡罗酸对DMSO的氧化。 反应量热测试还表明,即使在40℃下DMSO的氧化速率也很快,并且DMSO氧化的绝热温升高于400 K。
更新日期:2024-05-24
中文翻译:

50% H2O2/H2SO4 溶液与一些常见有机溶剂的相容性研究
在本文中,使用硫酸和50%过氧化氢溶液的混合物来生产卡罗酸,它是精细化工行业中常见的氧化剂。 50% H 2 O 2 /H 2 SO 4 与有机溶剂之间的化学相容性是一个重要的安全问题。 50% H 2 O 2 /H 2 SO 4 与多种有机溶剂(包括乙醇、乙腈、乙酸乙酯)的相容性、DMF、二氯乙烷、DMSO、乙醚和甲苯,通过反应量热法和热分析技术进行了研究。结果发现,前5种溶剂与50% H 2 O 2 /H 2 SO 4 表现出良好的相容性。 DSC测试表明,这五种溶剂在发生前几乎不与50% H 2 O 2 /H 2 SO 4 发生反应50% H 2 O 2 /H 2 SO 4 的分解温度 (∼93.3 °C)。 RADEX 测试表明,50% H 2 O 2 /H 2 SO 4 与这些溶剂的反应释放热量从 800 到1100 J g –1 ,压力对这些混合物的影响很明显。相比之下,后三种溶剂,即 DMSO、乙醚和甲苯,与 50% H 2 O 2 /H 2 SO 4 ,在较低温度下表现出显着的放热信号。最有趣的是,DMSO和50% H 2 O 2 /H 2 SO 4 的混合物在低于40℃时出现热失控现象°C。此次热失控事件的原因被确认为卡罗酸对DMSO的氧化。 反应量热测试还表明,即使在40℃下DMSO的氧化速率也很快,并且DMSO氧化的绝热温升高于400 K。






































 京公网安备 11010802027423号
京公网安备 11010802027423号