当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sedimentation kinetics and stability mechanisms of iron and manganese colloids in simulated groundwater
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2024-05-24 , DOI: 10.1039/d3en00811h Shuxin Huang 1 , Caixiang Zhang 1, 2 , Lu Chen 1 , Ruihan Xiong 1 , Jiasen Li 1 , Jidao Xie 1 , Zenghui Fan 1 , You Lv 1
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2024-05-24 , DOI: 10.1039/d3en00811h Shuxin Huang 1 , Caixiang Zhang 1, 2 , Lu Chen 1 , Ruihan Xiong 1 , Jiasen Li 1 , Jidao Xie 1 , Zenghui Fan 1 , You Lv 1
Affiliation
|
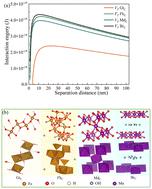
The occurrence of different forms of iron and manganese colloids in the subsurface environment has been widely reported. However, their sedimentation kinetics and stability mechanisms have been scarcely addressed. In this study, the effect of pH, electrolytes, and fulvic acid on the 24 h-scale sedimentation kinetics of iron and manganese colloids was investigated, and their mechanisms were described by the classic Derjaguin–Landau–Verwey–Overbeek (DLVO) and extended DLVO (XDLVO) theories. The results indicated that the colloidal sedimentation behavior was not only affected by their structures, but also by their hydrochemical environments. The sedimentation rate of these colloids increased with increasing pH. The electric double layer of colloids was compressed at a higher electrolyte concentration and valence state, further inducing colloidal sedimentation. However, fulvic acid could retard colloidal sedimentation as a result of electrostatic repulsion and a steric hindrance effect. The DLVO and XDLVO theory results suggest that both the total potential energy and the energy barrier decreased with increasing electrolyte concentration or valence state, whereas they increased with increasing fulvic acid concentration. Moreover, the repulsive potential was dominant for iron colloid sedimentation in Na+ and Ca2+, whereas the sedimentation of manganese colloids was mainly controlled by the repulsive potential in Na+ and the attractive potential in Ca2+. These findings provide a basic reference for understanding the fate and transport of iron and manganese colloids and associated contaminants in various subsurface environments.
中文翻译:

模拟地下水中铁锰胶体的沉降动力学和稳定机制
地下环境中不同形式的铁和锰胶体的存在已被广泛报道。然而,它们的沉降动力学和稳定性机制却很少得到解决。在这项研究中,研究了pH、电解质和黄腐酸对铁和锰胶体24小时尺度沉降动力学的影响,并通过经典的Derjaguin–Landau–Verwey–Overbeek (DLVO)描述了它们的机制并扩展了DLVO(XDLVO)理论。结果表明,胶体沉降行为不仅受到其结构的影响,还受到其水化学环境的影响。这些胶体的沉降速率随着pH值的增加而增加。在较高的电解质浓度和价态下,胶体的双电层被压缩,进一步诱导胶体沉降。然而,由于静电排斥和空间位阻效应,黄腐酸可以阻碍胶体沉降。 DLVO和XDLVO理论结果表明,总势能和能垒都随着电解质浓度或价态的增加而降低,而随着黄腐酸浓度的增加而增加。此外,Na + 和Ca 2+ 中铁胶体的沉降主要由排斥势控制,而锰胶体的沉降主要受Na + 中排斥势的控制。 b2> 和 Ca 2+ 中的吸引力潜力。这些发现为了解各种地下环境中铁锰胶体及相关污染物的归宿和迁移提供了基本参考。
更新日期:2024-05-24
中文翻译:

模拟地下水中铁锰胶体的沉降动力学和稳定机制
地下环境中不同形式的铁和锰胶体的存在已被广泛报道。然而,它们的沉降动力学和稳定性机制却很少得到解决。在这项研究中,研究了pH、电解质和黄腐酸对铁和锰胶体24小时尺度沉降动力学的影响,并通过经典的Derjaguin–Landau–Verwey–Overbeek (DLVO)描述了它们的机制并扩展了DLVO(XDLVO)理论。结果表明,胶体沉降行为不仅受到其结构的影响,还受到其水化学环境的影响。这些胶体的沉降速率随着pH值的增加而增加。在较高的电解质浓度和价态下,胶体的双电层被压缩,进一步诱导胶体沉降。然而,由于静电排斥和空间位阻效应,黄腐酸可以阻碍胶体沉降。 DLVO和XDLVO理论结果表明,总势能和能垒都随着电解质浓度或价态的增加而降低,而随着黄腐酸浓度的增加而增加。此外,Na + 和Ca 2+ 中铁胶体的沉降主要由排斥势控制,而锰胶体的沉降主要受Na + 中排斥势的控制。 b2> 和 Ca 2+ 中的吸引力潜力。这些发现为了解各种地下环境中铁锰胶体及相关污染物的归宿和迁移提供了基本参考。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号