当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solvent Switching in Continuous Multistep Chemoenzymatic Synthesis: Telescoping Enzymatic Synthesis of Chiral, Pyridine-Containing Amines with Cross-Coupling as a Case Study
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-05-21 , DOI: 10.1021/acs.oprd.4c00080 Pablo Díaz-Kruik 1 , David Roura Padrosa 2 , Eimear Hegarty 1 , Hansjoerg Lehmann 3 , Radka Snajdrova 3 , Francesca Paradisi 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-05-21 , DOI: 10.1021/acs.oprd.4c00080 Pablo Díaz-Kruik 1 , David Roura Padrosa 2 , Eimear Hegarty 1 , Hansjoerg Lehmann 3 , Radka Snajdrova 3 , Francesca Paradisi 1
Affiliation
|
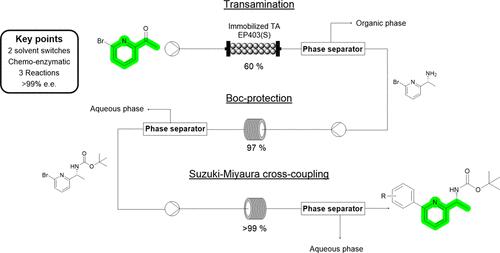
Chiral α-(hetero)aryl primary amines are gaining momentum for their biological activities and their use as building blocks in more complex molecules. Here we report a continuous chemoenzymatic strategy from 2-acetyl-6-bromopyridine enabled by careful solvent selection and phase switching. Combining a first biocatalytic transamination reaction performed by TsRTA in a biphasic system in continuous flow, with inline Boc-protection and Suzuki coupling of a (substituted)phenylboronic acid, achieves conversions up to >99% toward tert-butyl (R)-(1-(6-(substituted) phenylpyridin-2-yl)ethyl)carbamate as the final product. This strategy not only constitutes an important example of chemoenzymatic combinations in continuous flow but also highlights the importance of the reaction design to minimize waste (through unreacted substrate recirculation), avoid time intensive workups (through inline extractions), and achieve the product in a space time yield of 68 mg L–1 h–1 with excellent enantiomeric excess (99% ee).
中文翻译:

连续多步化学酶合成中的溶剂切换:以交叉偶联手性含吡啶胺的伸缩酶法合成为例
手性 α-(杂)芳基伯胺因其生物活性和作为更复杂分子的构建单元的用途而获得动力。在这里,我们报告了通过仔细的溶剂选择和相转换实现的 2-乙酰基-6-溴吡啶的连续化学酶策略。将 TsRTA 在连续流双相系统中进行的首次生物催化转氨反应与(取代)苯基硼酸的内联 Boc 保护和 Suzuki 偶联相结合,实现了高达 99% 以上的叔丁基 (R)-(1最终产物为-(6-(取代)苯基吡啶-2-基)乙基)氨基甲酸酯。该策略不仅构成了连续流中化学酶组合的重要示例,而且还强调了反应设计的重要性,以最大限度地减少浪费(通过未反应的底物再循环)、避免时间密集型后处理(通过在线提取)并在空间中获得产物时间产量为 68 mg L –1 h –1 ,具有出色的对映体过量 (99% ee)。
更新日期:2024-05-23
中文翻译:

连续多步化学酶合成中的溶剂切换:以交叉偶联手性含吡啶胺的伸缩酶法合成为例
手性 α-(杂)芳基伯胺因其生物活性和作为更复杂分子的构建单元的用途而获得动力。在这里,我们报告了通过仔细的溶剂选择和相转换实现的 2-乙酰基-6-溴吡啶的连续化学酶策略。将 TsRTA 在连续流双相系统中进行的首次生物催化转氨反应与(取代)苯基硼酸的内联 Boc 保护和 Suzuki 偶联相结合,实现了高达 99% 以上的叔丁基 (R)-(1最终产物为-(6-(取代)苯基吡啶-2-基)乙基)氨基甲酸酯。该策略不仅构成了连续流中化学酶组合的重要示例,而且还强调了反应设计的重要性,以最大限度地减少浪费(通过未反应的底物再循环)、避免时间密集型后处理(通过在线提取)并在空间中获得产物时间产量为 68 mg L –1 h –1 ,具有出色的对映体过量 (99% ee)。






































 京公网安备 11010802027423号
京公网安备 11010802027423号