当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ketoreductase-Mediated Stereodivergent Synthesis of 1,1-Disubstituted Cyclobutan-3-ols
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-05-21 , DOI: 10.1021/acs.oprd.4c00126 Hardwin O’Dowd 1 , Olivia M. Morales 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-05-21 , DOI: 10.1021/acs.oprd.4c00126 Hardwin O’Dowd 1 , Olivia M. Morales 1
Affiliation
|
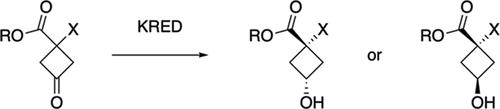
Selective access to both cis- and trans-isomers of α-substituted 3-hydroxycyclobutane-1-carboxylic esters has been demonstrated using ketoreductase (KRED) enzymes. Two stereocomplementary KREDs were identified from a commercial panel that are highly selective for small α-substituents when the ester group was para-methoxybenzyl. Probing the substrate scope of these two enzymes revealed that the selectivity declined rapidly with both when increasing the steric bulk of the α-substituent. For methyl ester substrates with larger α-substituents, a different pair of KREDs was identified with excellent selectivity for both the cis- and trans-isomers for a small range of substrates. The cyclobutanol products serve as precursors to other synthetically useful building blocks such as amino acids.
中文翻译:

酮还原酶介导的 1,1-二取代环丁烷-3-醇的立体发散合成
使用酮还原酶 (KRED) 已证明可以选择性地获得 α-取代的 3-羟基环丁烷-1-羧酸酯的顺式和反式异构体。从商业小组中鉴定出两种立体互补的 KRED,当酯基为对甲氧基苄基时,它们对小 α-取代基具有高度选择性。探索这两种酶的底物范围表明,当增加 α-取代基的空间体积时,两者的选择性迅速下降。对于具有较大 α-取代基的甲酯底物,鉴定出一对不同的 KRED,对小范围底物的顺式和反式异构体具有出色的选择性。环丁醇产品可作为其他合成有用的结构单元(例如氨基酸)的前体。
更新日期:2024-05-23
中文翻译:

酮还原酶介导的 1,1-二取代环丁烷-3-醇的立体发散合成
使用酮还原酶 (KRED) 已证明可以选择性地获得 α-取代的 3-羟基环丁烷-1-羧酸酯的顺式和反式异构体。从商业小组中鉴定出两种立体互补的 KRED,当酯基为对甲氧基苄基时,它们对小 α-取代基具有高度选择性。探索这两种酶的底物范围表明,当增加 α-取代基的空间体积时,两者的选择性迅速下降。对于具有较大 α-取代基的甲酯底物,鉴定出一对不同的 KRED,对小范围底物的顺式和反式异构体具有出色的选择性。环丁醇产品可作为其他合成有用的结构单元(例如氨基酸)的前体。






































 京公网安备 11010802027423号
京公网安备 11010802027423号