当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The flavohemoglobin Yhb1 is a new interacting partner of the heme transporter Str3
Molecular Microbiology ( IF 2.6 ) Pub Date : 2024-05-22 , DOI: 10.1111/mmi.15281 Florie Lo Ying Ping 1 , Tobias Vahsen 1 , Ariane Brault 1 , Raphaël Néré 1 , Simon Labbé 1
Molecular Microbiology ( IF 2.6 ) Pub Date : 2024-05-22 , DOI: 10.1111/mmi.15281 Florie Lo Ying Ping 1 , Tobias Vahsen 1 , Ariane Brault 1 , Raphaël Néré 1 , Simon Labbé 1
Affiliation
|
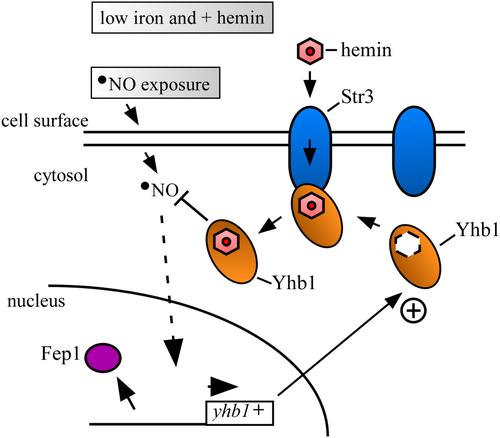
Nitric oxide (˙NO) is a free radical that induces nitrosative stress, which can jeopardize cell viability. Yeasts have evolved diverse detoxification mechanisms to effectively counteract ˙NO-mediated cytotoxicity. One mechanism relies on the flavohemoglobin Yhb1, whereas a second one requires the S-nitrosoglutathione reductase Fmd2. To investigate heme-dependent activation of Yhb1 in response to ˙NO, we use hem1Δ-derivative Schizosaccharomyces pombe strains lacking the initial enzyme in heme biosynthesis, forcing cells to assimilate heme from external sources. Under these conditions, yhb1+ mRNA levels are repressed in the presence of iron through a mechanism involving the GATA-type transcriptional repressor Fep1. In contrast, when iron levels are low, the transcription of yhb1+ is derepressed and further induced in the presence of the ˙NO donor DETANONOate. Cells lacking Yhb1 or expressing inactive forms of Yhb1 fail to grow in a hemin-dependent manner when exposed to DETANONOate. Similarly, the loss of function of the heme transporter Str3 phenocopies the effects of Yhb1 disruption by causing hypersensitivity to DETANONOate under hemin-dependent culture conditions. Coimmunoprecipitation and bimolecular fluorescence complementation assays demonstrate the interaction between Yhb1 and the heme transporter Str3. Collectively, our findings unveil a novel pathway for activating Yhb1, fortifying yeast cells against nitrosative stress.
中文翻译:

黄素血红蛋白 Yhb1 是血红素转运蛋白 Str3 的新相互作用伙伴
一氧化氮 (NO) 是一种自由基,可引起亚硝化应激,从而危及细胞活力。酵母已经进化出多种解毒机制来有效抵消一氧化氮介导的细胞毒性。一种机制依赖于黄素血红蛋白 Yhb1,而第二种机制则需要 S-亚硝基谷胱甘肽还原酶 Fmd2。为了研究 Yhb1 响应 ˙NO 的血红素依赖性激活,我们使用了 hem1Δ 衍生的粟酒裂殖酵母菌株,该菌株缺乏血红素生物合成中的初始酶,迫使细胞从外部来源同化血红素。在这些条件下,yhb1 + mRNA 水平在铁存在下通过涉及 GATA 型转录抑制子 Fep1 的机制受到抑制。相反,当铁水平较低时,yhb1 + 的转录受到抑制,并在 ˙NO 供体 DETANONOate 的存在下进一步被诱导。当暴露于 DETANONOate 时,缺乏 Yhb1 或表达非活性形式 Yhb1 的细胞无法以血红素依赖性方式生长。同样,血红素转运蛋白 Str3 功能的丧失通过在血红素依赖性培养条件下引起对 DETANONOate 的超敏反应来表现 Yhb1 破坏的影响。免疫共沉淀和双分子荧光互补测定证明了 Yhb1 和血红素转运蛋白 Str3 之间的相互作用。总的来说,我们的研究结果揭示了一种激活 Yhb1 的新途径,可增强酵母细胞抵抗亚硝化应激的能力。
更新日期:2024-05-22
中文翻译:

黄素血红蛋白 Yhb1 是血红素转运蛋白 Str3 的新相互作用伙伴
一氧化氮 (NO) 是一种自由基,可引起亚硝化应激,从而危及细胞活力。酵母已经进化出多种解毒机制来有效抵消一氧化氮介导的细胞毒性。一种机制依赖于黄素血红蛋白 Yhb1,而第二种机制则需要 S-亚硝基谷胱甘肽还原酶 Fmd2。为了研究 Yhb1 响应 ˙NO 的血红素依赖性激活,我们使用了 hem1Δ 衍生的粟酒裂殖酵母菌株,该菌株缺乏血红素生物合成中的初始酶,迫使细胞从外部来源同化血红素。在这些条件下,yhb1 + mRNA 水平在铁存在下通过涉及 GATA 型转录抑制子 Fep1 的机制受到抑制。相反,当铁水平较低时,yhb1 + 的转录受到抑制,并在 ˙NO 供体 DETANONOate 的存在下进一步被诱导。当暴露于 DETANONOate 时,缺乏 Yhb1 或表达非活性形式 Yhb1 的细胞无法以血红素依赖性方式生长。同样,血红素转运蛋白 Str3 功能的丧失通过在血红素依赖性培养条件下引起对 DETANONOate 的超敏反应来表现 Yhb1 破坏的影响。免疫共沉淀和双分子荧光互补测定证明了 Yhb1 和血红素转运蛋白 Str3 之间的相互作用。总的来说,我们的研究结果揭示了一种激活 Yhb1 的新途径,可增强酵母细胞抵抗亚硝化应激的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号