当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iodine-Catalyzed Cyclization of o-Nitrothiophenols with Cyclohexanones to Phenothiazines
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-05-21 , DOI: 10.1021/acs.joc.4c00039 Yinglin Zhao 1 , Jin Zhang 1 , Jingwu Zhang 2 , Zhida Zhang 1 , Renhua Liu 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-05-21 , DOI: 10.1021/acs.joc.4c00039 Yinglin Zhao 1 , Jin Zhang 1 , Jingwu Zhang 2 , Zhida Zhang 1 , Renhua Liu 1
Affiliation
|
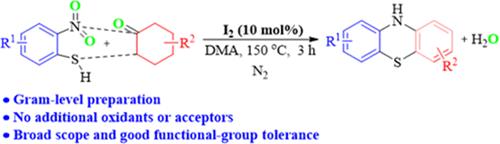
Here, a novel iodine-catalyzed direct cyclization of o-nitrothiophenols with cyclohexanones to phenothiazines has been described without external oxidants and hydrogen acceptors. The nitro of o-nitrothiophenol works as both a hydrogen acceptor and a coupling group, and water is the only byproduct. The reaction involves the reduction of nitro groups, C–H bond thioetherification, and C–H bond dehydroaromatization. This scheme offers broad synthetic value for further elaborations, as exemplified by a 3-step total synthesis of antipsychotic chlorpromazine.
中文翻译:

碘催化邻硝基苯硫酚与环己酮环化生成吩噻嗪
在此,描述了一种新型的碘催化邻硝基苯硫酚与环己酮直接环化为吩噻嗪的方法,无需外部氧化剂和氢受体。邻硝基苯硫酚的硝基既充当氢受体又充当偶联基团,水是唯一的副产物。该反应涉及硝基还原、C-H键硫醚化和C-H键脱氢芳构化。该方案为进一步阐述提供了广泛的合成价值,例如抗精神病药氯丙嗪的三步全合成。
更新日期:2024-05-21
中文翻译:

碘催化邻硝基苯硫酚与环己酮环化生成吩噻嗪
在此,描述了一种新型的碘催化邻硝基苯硫酚与环己酮直接环化为吩噻嗪的方法,无需外部氧化剂和氢受体。邻硝基苯硫酚的硝基既充当氢受体又充当偶联基团,水是唯一的副产物。该反应涉及硝基还原、C-H键硫醚化和C-H键脱氢芳构化。该方案为进一步阐述提供了广泛的合成价值,例如抗精神病药氯丙嗪的三步全合成。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号