当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
High-fat diet induces sarcopenic obesity in natural aging rats through the gut–trimethylamine N-oxide–muscle axis
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-05-12 , DOI: 10.1016/j.jare.2024.05.015 Xiaoxing Mo , Ruijie Cheng , Lihui Shen , Yunhong Sun , Pei Wang , Guanhua Jiang , Lin Wen , Xiaoqin Li , Xiaobo Peng , Yuxiao Liao , Ruikun He , Hong Yan , Liegang Liu
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-05-12 , DOI: 10.1016/j.jare.2024.05.015 Xiaoxing Mo , Ruijie Cheng , Lihui Shen , Yunhong Sun , Pei Wang , Guanhua Jiang , Lin Wen , Xiaoqin Li , Xiaobo Peng , Yuxiao Liao , Ruikun He , Hong Yan , Liegang Liu
|
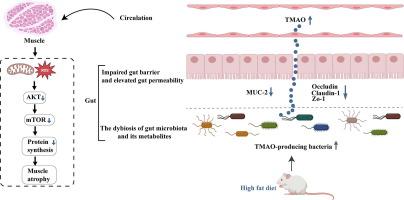
The lack of suitable animal models for sarcopenic obesity (SO) limits in-depth research into the disease. Emerging studies have demonstrated that gut dysbiosis is involved in the development of SO. As the importance of microbial metabolites is starting to unveil, it is necessary to comprehend the specific metabolites associated with gut microbiota and SO. We aimed to investigate whether high-fat diet (HFD) causes SO in natural aging animal models and specific microbial metabolites that are involved in linking HFD and SO. Young rats received HFD or control diet for 80 weeks, and obesity-related metabolic disorders and sarcopenia were measured. 16S rRNA sequencing and non-targeted and targeted metabolomics methods were used to detect fecal gut microbiota and serum metabolites. Gut barrier function was evaluated by intestinal barrier integrity and intestinal permeability. Trimethylamine N-oxide (TMAO) treatment was further conducted for verification. HFD resulted in body weight gain, dyslipidemia, impaired glucose tolerance, insulin resistance, and systemic inflammation in natural aging rats. HFD also caused decreases in muscle mass, strength, function, and fiber cross-sectional area and increase in muscle fatty infiltration in natural aging rats. 16S rRNA sequencing and nontargeted and targeted metabolomics analysis indicated that HFD contributed to gut dysbiosis, mainly characterized by increases in deleterious bacteria and TMAO. HFD destroyed intestinal barrier integrity and increased intestinal permeability, as evaluated by reducing levels of colonic mucin-2, tight junction proteins, goblet cells and elevating serum level of fluorescein isothiocyanate-dextran 4. Correlation analysis showed a positive association between TMAO and SO. In addition, TMAO treatment aggravated the development of SO in HFD-fed aged rats through regulating the ROS-AKT/mTOR signaling pathway. HFD leads to SO in natural aging rats, partially through the gut–microbiota–TMAO–muscle axis.
中文翻译:

高脂饮食通过肠道-三甲胺N-氧化物-肌肉轴诱导自然衰老大鼠肌肉减少性肥胖
缺乏合适的少肌性肥胖(SO)动物模型限制了对该疾病的深入研究。新兴研究表明肠道菌群失调与 SO 的发生有关。随着微生物代谢物的重要性开始显现,有必要了解与肠道微生物群和 SO 相关的特定代谢物。我们的目的是研究高脂饮食 (HFD) 是否会在自然衰老的动物模型中导致 SO,以及参与连接 HFD 和 SO 的特定微生物代谢物。幼鼠接受 HFD 或对照饮食 80 周,并测量与肥胖相关的代谢紊乱和肌肉减少症。采用16S rRNA测序以及非靶向和靶向代谢组学方法来检测粪便肠道微生物群和血清代谢物。肠道屏障功能通过肠道屏障完整性和肠道通透性进行评估。进一步进行三甲胺N-氧化物(TMAO)处理进行验证。 HFD 导致自然衰老大鼠体重增加、血脂异常、糖耐量受损、胰岛素抵抗和全身炎症。 HFD 还会导致自然衰老大鼠的肌肉质量、力量、功能和纤维横截面积下降,以及肌肉脂肪浸润增加。 16S rRNA测序以及非靶向和靶向代谢组学分析表明,HFD导致肠道菌群失调,主要表现为有害细菌和TMAO增加。通过降低结肠粘蛋白-2、紧密连接蛋白、杯状细胞的水平和提高异硫氰酸荧光素-葡聚糖 4 的血清水平来评估,HFD 破坏了肠道屏障完整性并增加了肠道通透性。相关分析显示 TMAO 与 SO 之间呈正相关。 此外,TMAO治疗通过调节ROS-AKT/mTOR信号通路,加剧了高脂饮食老年大鼠SO的发生。 HFD 在自然衰老的大鼠中导致 SO,部分是通过肠道-微生物群-TMAO-肌肉轴。
更新日期:2024-05-12
中文翻译:

高脂饮食通过肠道-三甲胺N-氧化物-肌肉轴诱导自然衰老大鼠肌肉减少性肥胖
缺乏合适的少肌性肥胖(SO)动物模型限制了对该疾病的深入研究。新兴研究表明肠道菌群失调与 SO 的发生有关。随着微生物代谢物的重要性开始显现,有必要了解与肠道微生物群和 SO 相关的特定代谢物。我们的目的是研究高脂饮食 (HFD) 是否会在自然衰老的动物模型中导致 SO,以及参与连接 HFD 和 SO 的特定微生物代谢物。幼鼠接受 HFD 或对照饮食 80 周,并测量与肥胖相关的代谢紊乱和肌肉减少症。采用16S rRNA测序以及非靶向和靶向代谢组学方法来检测粪便肠道微生物群和血清代谢物。肠道屏障功能通过肠道屏障完整性和肠道通透性进行评估。进一步进行三甲胺N-氧化物(TMAO)处理进行验证。 HFD 导致自然衰老大鼠体重增加、血脂异常、糖耐量受损、胰岛素抵抗和全身炎症。 HFD 还会导致自然衰老大鼠的肌肉质量、力量、功能和纤维横截面积下降,以及肌肉脂肪浸润增加。 16S rRNA测序以及非靶向和靶向代谢组学分析表明,HFD导致肠道菌群失调,主要表现为有害细菌和TMAO增加。通过降低结肠粘蛋白-2、紧密连接蛋白、杯状细胞的水平和提高异硫氰酸荧光素-葡聚糖 4 的血清水平来评估,HFD 破坏了肠道屏障完整性并增加了肠道通透性。相关分析显示 TMAO 与 SO 之间呈正相关。 此外,TMAO治疗通过调节ROS-AKT/mTOR信号通路,加剧了高脂饮食老年大鼠SO的发生。 HFD 在自然衰老的大鼠中导致 SO,部分是通过肠道-微生物群-TMAO-肌肉轴。











































 京公网安备 11010802027423号
京公网安备 11010802027423号