当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
MIER2/PGC1A elicits sunitinib resistance via lipid metabolism in renal cell carcinoma
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-05-01 , DOI: 10.1016/j.jare.2024.04.032 Zhihao Wei , Yuzhong Ye , Chenchen Liu , Qi Wang , Yunxuan Zhang , Kailei Chen , Gong Cheng , Xiaoping Zhang
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-05-01 , DOI: 10.1016/j.jare.2024.04.032 Zhihao Wei , Yuzhong Ye , Chenchen Liu , Qi Wang , Yunxuan Zhang , Kailei Chen , Gong Cheng , Xiaoping Zhang
|
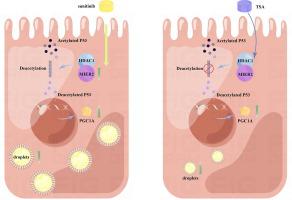
Renal cell carcinoma (RCC) is one of the most common malignant tumors of the urinary system and accounts for more than 90 % of all renal tumors. Resistance to targeted therapy has emerged as a pivotal factor that contributes to the progressive deterioration of patients with advanced RCC. Metabolic reprogramming is a hallmark of tumorigenesis and progression, with an increasing body of evidence indicating that abnormal lipid metabolism plays a crucial role in the advancement of renal clear cell carcinoma. Clarify the precise mechanisms underlying abnormal lipid metabolism and drug resistance. Bioinformatics screening and analyses were performed to identify hub gene. qRT-PCR, western blot, chromatin immunoprecipitation (ChIP) assays, and other biological methods were used to explore and verify related pathways. Various cell line models and animal models were used to perform biological functional experiments. In this study, we identified Mesoderm induction early response 2 (MIER2) as a novel biomarker for RCC, demonstrating its role in promoting malignancy and sunitinib resistance by influencing lipid metabolism in RCC. Mechanistically, MIER2 facilitated P53 deacetylation by binding to HDAC1. Acetylation modification augmented the DNA-binding stability and transcriptional function of P53, while deacetylation of P53 hindered the transcriptional process of PGC1A, leading to intracellular lipid accumulation in RCC. Furthermore, Trichostatin A (TSA), an inhibitor of HDAC1, was found to impede the MIER2/HDAC1/P53/PGC1A pathway, offering potential benefits for patients with sunitinib-resistant renal cell cancer. Our findings highlight MIER2 as a key player in anchoring HDAC1 and inhibiting PGC1A expression through the deacetylation of P53, thereby inducing lipid accumulation in RCC and promoting drug resistance. Lipid-rich RCC cells compensate for energy production and sustain their own growth in a glycolysis-independent manner, evading the cytotoxic effects of targeted drugs and ultimately culminating in the development of drug resistance.
中文翻译:

MIER2/PGC1A 通过肾细胞癌的脂质代谢引发舒尼替尼耐药
肾细胞癌(RCC)是泌尿系统最常见的恶性肿瘤之一,占所有肾肿瘤的90%以上。对靶向治疗的耐药性已成为导致晚期肾细胞癌患者病情进行性恶化的关键因素。代谢重编程是肿瘤发生和进展的标志,越来越多的证据表明脂质代谢异常在肾透明细胞癌的进展中起着至关重要的作用。阐明脂质代谢异常和耐药性的确切机制。进行生物信息学筛选和分析以鉴定hub基因。采用qRT-PCR、蛋白质印迹、染色质免疫沉淀(ChIP)测定和其他生物学方法来探索和验证相关通路。采用各种细胞系模型和动物模型进行生物功能实验。在这项研究中,我们将中胚层诱导早期反应 2 (MIER2) 确定为 RCC 的新型生物标志物,证明其通过影响 RCC 脂质代谢而促进恶性肿瘤和舒尼替尼耐药的作用。从机制上讲,MIER2 通过与 HDAC1 结合促进 P53 脱乙酰化。乙酰化修饰增强了 P53 的 DNA 结合稳定性和转录功能,而 P53 的去乙酰化则阻碍了 PGC1A 的转录过程,导致 RCC 细胞内脂质积累。此外,HDAC1 抑制剂曲古抑菌素 A (TSA) 被发现可阻碍 MIER2/HDAC1/P53/PGC1A 通路,为舒尼替尼耐药的肾细胞癌患者带来潜在益处。 我们的研究结果强调 MIER2 作为锚定 HDAC1 和通过 P53 脱乙酰化抑制 PGC1A 表达的关键角色,从而诱导 RCC 中的脂质积累并促进耐药性。富含脂质的肾细胞癌细胞补偿能量产生,并以不依赖糖酵解的方式维持自身生长,逃避靶向药物的细胞毒性作用,最终导致耐药性的发展。
更新日期:2024-05-01
中文翻译:

MIER2/PGC1A 通过肾细胞癌的脂质代谢引发舒尼替尼耐药
肾细胞癌(RCC)是泌尿系统最常见的恶性肿瘤之一,占所有肾肿瘤的90%以上。对靶向治疗的耐药性已成为导致晚期肾细胞癌患者病情进行性恶化的关键因素。代谢重编程是肿瘤发生和进展的标志,越来越多的证据表明脂质代谢异常在肾透明细胞癌的进展中起着至关重要的作用。阐明脂质代谢异常和耐药性的确切机制。进行生物信息学筛选和分析以鉴定hub基因。采用qRT-PCR、蛋白质印迹、染色质免疫沉淀(ChIP)测定和其他生物学方法来探索和验证相关通路。采用各种细胞系模型和动物模型进行生物功能实验。在这项研究中,我们将中胚层诱导早期反应 2 (MIER2) 确定为 RCC 的新型生物标志物,证明其通过影响 RCC 脂质代谢而促进恶性肿瘤和舒尼替尼耐药的作用。从机制上讲,MIER2 通过与 HDAC1 结合促进 P53 脱乙酰化。乙酰化修饰增强了 P53 的 DNA 结合稳定性和转录功能,而 P53 的去乙酰化则阻碍了 PGC1A 的转录过程,导致 RCC 细胞内脂质积累。此外,HDAC1 抑制剂曲古抑菌素 A (TSA) 被发现可阻碍 MIER2/HDAC1/P53/PGC1A 通路,为舒尼替尼耐药的肾细胞癌患者带来潜在益处。 我们的研究结果强调 MIER2 作为锚定 HDAC1 和通过 P53 脱乙酰化抑制 PGC1A 表达的关键角色,从而诱导 RCC 中的脂质积累并促进耐药性。富含脂质的肾细胞癌细胞补偿能量产生,并以不依赖糖酵解的方式维持自身生长,逃避靶向药物的细胞毒性作用,最终导致耐药性的发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号