当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sodium-glucose exchanger 2 inhibitor canagliflozin promotes mitochondrial metabolism and alleviates salt-induced cardiac hypertrophy via preserving SIRT3 expression
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-05-12 , DOI: 10.1016/j.jare.2024.04.030 Yu Zhao , Zongshi Lu , Hexuan Zhang , Lijuan Wang , Fang Sun , Qiang Li , Tingbing Cao , Bowen Wang , Huan Ma , Mei You , Qing Zhou , Xiao Wei , Li Li , Yingying Liao , Zhencheng Yan , Daoyan Liu , Peng Gao , Zhiming Zhu
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-05-12 , DOI: 10.1016/j.jare.2024.04.030 Yu Zhao , Zongshi Lu , Hexuan Zhang , Lijuan Wang , Fang Sun , Qiang Li , Tingbing Cao , Bowen Wang , Huan Ma , Mei You , Qing Zhou , Xiao Wei , Li Li , Yingying Liao , Zhencheng Yan , Daoyan Liu , Peng Gao , Zhiming Zhu
|
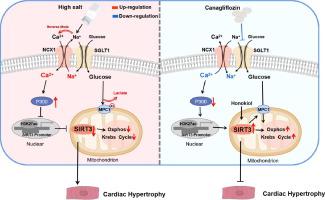
Excess salt intake is not only an independent risk factor for heart failure, but also one of the most important dietary factors associated with cardiovascular disease worldwide. Metabolic reprogramming in cardiomyocytes is an early event provoking cardiac hypertrophy that leads to subsequent cardiovascular events upon high salt loading. Although SGLT2 inhibitors, such as canagliflozin, displayed impressive cardiovascular health benefits, whether SGLT2 inhibitors protect against cardiac hypertrophy-related metabolic reprogramming upon salt loading remain elusive. To investigate whether canagliflozin can improve salt-induced cardiac hypertrophy and the underlying mechanisms. Dahl salt-sensitive rats developed cardiac hypertrophy by feeding them an 8% high-salt diet, and some rats were treated with canagliflozin. Cardiac function and structure as well as mitochondrial function were examined. Cardiac proteomics, targeted metabolomics and SIRT3 cardiac-specific knockout mice were used to uncover the underlying mechanisms. In Dahl salt-sensitive rats, canagliflozin showed a potent therapeutic effect on salt-induced cardiac hypertrophy, accompanied by lowered glucose uptake, reduced accumulation of glycolytic end-products and improved cardiac mitochondrial function, which was associated with the recovery of cardiac expression of SIRT3, a key mitochondrial metabolic regulator. Cardiac-specific knockout of SIRT3 not only exacerbated salt-induced cardiac hypertrophy but also abolished the therapeutic effect of canagliflozin. Mechanistically, high salt intake repressed cardiac SIRT3 expression through a calcium-dependent epigenetic modifications, which could be blocked by canagliflozin by inhibiting SGLT1-mediated calcium uptake. SIRT3 improved myocardial metabolic reprogramming by deacetylating MPC1 in cardiomyocytes exposed to pro-hypertrophic stimuli. Similar to canagliflozin, the SIRT3 activator honokiol also exerted therapeutic effects on cardiac hypertrophy. Cardiac mitochondrial dysfunction caused by SIRT3 repression is a critical promotional determinant of metabolic pattern switching underlying salt-induced cardiac hypertrophy. Improving SIRT3-mediated mitochondrial function by SGLT2 inhibitors-mediated calcium handling would represent a therapeutic strategy against salt-related cardiovascular events.
中文翻译:

钠-葡萄糖交换器 2 抑制剂卡格列净通过保留 SIRT3 表达促进线粒体代谢并减轻盐诱导的心脏肥大
过量盐摄入不仅是心力衰竭的独立危险因素,而且是全世界与心血管疾病相关的最重要的饮食因素之一。心肌细胞中的代谢重编程是引发心脏肥大的早期事件,在高盐负荷下导致随后的心血管事件。尽管 SGLT2 抑制剂(例如卡格列净)显示出令人印象深刻的心血管健康益处,但 SGLT2 抑制剂是否能防止盐负荷时心脏肥大相关的代谢重编程仍然难以捉摸。探讨卡格列净是否可以改善盐诱导的心肌肥厚及其潜在机制。 Dahl 对盐敏感的大鼠通过喂食 8% 的高盐饮食而出现心脏肥大,部分大鼠接受了卡格列净治疗。检查心脏功能和结构以及线粒体功能。使用心脏蛋白质组学、靶向代谢组学和 SIRT3 心脏特异性敲除小鼠来揭示潜在机制。在 Dahl 盐敏感大鼠中,卡格列净对盐诱导的心脏肥大显示出有效的治疗作用,同时降低葡萄糖摄取,减少糖酵解终产物的积累并改善心脏线粒体功能,这与 SIRT3 心脏表达的恢复有关,一种关键的线粒体代谢调节剂。心脏特异性敲除SIRT3不仅加剧了盐诱导的心脏肥大,而且消除了卡格列净的治疗作用。从机制上讲,高盐摄入通过钙依赖性表观遗传修饰抑制心脏 SIRT3 表达,卡格列净可以通过抑制 SGLT1 介导的钙摄取来阻断这种修饰。 SIRT3 通过使暴露于促肥大刺激的心肌细胞中的 MPC1 去乙酰化来改善心肌代谢重编程。与卡格列净类似,SIRT3激活剂和厚朴酚也对心脏肥大发挥治疗作用。 SIRT3 抑制引起的心脏线粒体功能障碍是盐诱导心脏肥大代谢模式转换的关键促进决定因素。通过 SGLT2 抑制剂介导的钙处理来改善 SIRT3 介导的线粒体功能将代表针对盐相关心血管事件的治疗策略。
更新日期:2024-05-12
中文翻译:

钠-葡萄糖交换器 2 抑制剂卡格列净通过保留 SIRT3 表达促进线粒体代谢并减轻盐诱导的心脏肥大
过量盐摄入不仅是心力衰竭的独立危险因素,而且是全世界与心血管疾病相关的最重要的饮食因素之一。心肌细胞中的代谢重编程是引发心脏肥大的早期事件,在高盐负荷下导致随后的心血管事件。尽管 SGLT2 抑制剂(例如卡格列净)显示出令人印象深刻的心血管健康益处,但 SGLT2 抑制剂是否能防止盐负荷时心脏肥大相关的代谢重编程仍然难以捉摸。探讨卡格列净是否可以改善盐诱导的心肌肥厚及其潜在机制。 Dahl 对盐敏感的大鼠通过喂食 8% 的高盐饮食而出现心脏肥大,部分大鼠接受了卡格列净治疗。检查心脏功能和结构以及线粒体功能。使用心脏蛋白质组学、靶向代谢组学和 SIRT3 心脏特异性敲除小鼠来揭示潜在机制。在 Dahl 盐敏感大鼠中,卡格列净对盐诱导的心脏肥大显示出有效的治疗作用,同时降低葡萄糖摄取,减少糖酵解终产物的积累并改善心脏线粒体功能,这与 SIRT3 心脏表达的恢复有关,一种关键的线粒体代谢调节剂。心脏特异性敲除SIRT3不仅加剧了盐诱导的心脏肥大,而且消除了卡格列净的治疗作用。从机制上讲,高盐摄入通过钙依赖性表观遗传修饰抑制心脏 SIRT3 表达,卡格列净可以通过抑制 SGLT1 介导的钙摄取来阻断这种修饰。 SIRT3 通过使暴露于促肥大刺激的心肌细胞中的 MPC1 去乙酰化来改善心肌代谢重编程。与卡格列净类似,SIRT3激活剂和厚朴酚也对心脏肥大发挥治疗作用。 SIRT3 抑制引起的心脏线粒体功能障碍是盐诱导心脏肥大代谢模式转换的关键促进决定因素。通过 SGLT2 抑制剂介导的钙处理来改善 SIRT3 介导的线粒体功能将代表针对盐相关心血管事件的治疗策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号