当前位置:
X-MOL 学术
›
Redox Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Iron accumulation in ovarian microenvironment damages the local redox balance and oocyte quality in aging mice
Redox Biology ( IF 10.7 ) Pub Date : 2024-05-17 , DOI: 10.1016/j.redox.2024.103195 Ye Chen , Jiaqi Zhang , Ying Tian , Xiangning Xu , Bicheng Wang , Ziqi Huang , Shuo Lou , Jingyi Kang , Ningning Zhang , Jing Weng , Yuanjing Liang , Wei Ma
Redox Biology ( IF 10.7 ) Pub Date : 2024-05-17 , DOI: 10.1016/j.redox.2024.103195 Ye Chen , Jiaqi Zhang , Ying Tian , Xiangning Xu , Bicheng Wang , Ziqi Huang , Shuo Lou , Jingyi Kang , Ningning Zhang , Jing Weng , Yuanjing Liang , Wei Ma
|
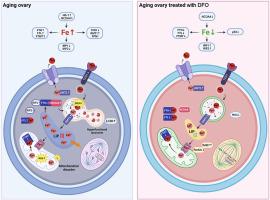
Accumulating oxidative damage is a primary driver of ovarian reserve decline along with aging. However, the mechanism behind the imbalance in reactive oxygen species (ROS) is not yet fully understood. Here we investigated changes in iron metabolism and its relationship with ROS disorder in aging ovaries of mice. We found increased iron content in aging ovaries and oocytes, along with abnormal expression of iron metabolic proteins, including heme oxygenase 1 (HO-1), ferritin heavy chain (FTH), ferritin light chain (FTL), mitochondrial ferritin (FTMT), divalent metal transporter 1 (DMT1), ferroportin1(FPN1), iron regulatory proteins (IRP1 and IRP2) and transferrin receptor 1 (TFR1). Notably, aging oocytes exhibited enhanced ferritinophagy and mitophagy, and consistently, there was an increase in cytosolic Fe2+, elevated lipid peroxidation, mitochondrial dysfunction, and augmented lysosome activity. Additionally, the ovarian expression of p53, p21, p16 and microtubule-associated protein tau (Tau) were also found to be upregulated. These alterations could be phenocopied with in vitro Fe2+ administration in oocytes from 2-month-old mice but were alleviated by deferoxamine (DFO). In vivo application of DFO improved ovarian iron metabolism and redox status in 12-month-old mice, and corrected the alterations in cytosolic Fe, ferritinophagy and mitophagy, as well as related degenerative changes in oocytes. Thereby in the whole, DFO delayed the decline in ovarian reserve and significantly increased the number of superovulated oocytes with reduced fragmentation and aneuploidy. Together, our findings suggest that aging-related disturbance in ovarian iron homeostasis contributes to excessive ROS production and that iron chelation may improve ovarian redox status, and efficiently delay the decline in ovarian reserve and oocyte quality in aging mice. These data propose a novel intervention strategy for preserving the ovarian reserve function in elderly women.
中文翻译:

卵巢微环境中铁的积累损害了衰老小鼠的局部氧化还原平衡和卵母细胞质量
累积的氧化损伤是卵巢储备随着衰老而下降的主要驱动因素。然而,活性氧(ROS)失衡背后的机制尚未完全清楚。在这里,我们研究了衰老小鼠卵巢中铁代谢的变化及其与ROS紊乱的关系。我们发现衰老的卵巢和卵母细胞中铁含量增加,同时铁代谢蛋白表达异常,包括血红素加氧酶 1 (HO-1)、铁蛋白重链 (FTH)、铁蛋白轻链 (FTL)、线粒体铁蛋白 (FTMT)、二价金属转运蛋白 1 (DMT1)、铁转运蛋白 1 (FPN1)、铁调节蛋白(IRP1 和 IRP2)和转铁蛋白受体 1 (TFR1)。值得注意的是,衰老的卵母细胞表现出铁蛋白自噬和线粒体自噬增强,并且胞质 Fe2+ 增加、脂质过氧化升高、线粒体功能障碍和溶酶体活性增强。此外,p53、p21、p16 和微管相关蛋白 tau (Tau) 的卵巢表达也被发现上调。这些改变可以通过体外给予 2 个月大小鼠的卵母细胞 Fe2+ 来进行表型复制,但可以通过去铁胺 (DFO) 得到缓解。 DFO的体内应用改善了12月龄小鼠的卵巢铁代谢和氧化还原状态,并纠正了胞浆Fe、铁蛋白自噬和线粒体自噬的改变以及卵母细胞的相关退行性变化。因此,总体而言,DFO 延缓了卵巢储备的下降,并显着增加了超排卵母细胞的数量,同时减少了碎片和非整倍体。 总之,我们的研究结果表明,与衰老相关的卵巢铁稳态紊乱导致ROS产生过多,而铁螯合可以改善卵巢氧化还原状态,并有效延缓衰老小鼠卵巢储备和卵母细胞质量的下降。这些数据提出了一种保护老年女性卵巢储备功能的新干预策略。
更新日期:2024-05-17
中文翻译:

卵巢微环境中铁的积累损害了衰老小鼠的局部氧化还原平衡和卵母细胞质量
累积的氧化损伤是卵巢储备随着衰老而下降的主要驱动因素。然而,活性氧(ROS)失衡背后的机制尚未完全清楚。在这里,我们研究了衰老小鼠卵巢中铁代谢的变化及其与ROS紊乱的关系。我们发现衰老的卵巢和卵母细胞中铁含量增加,同时铁代谢蛋白表达异常,包括血红素加氧酶 1 (HO-1)、铁蛋白重链 (FTH)、铁蛋白轻链 (FTL)、线粒体铁蛋白 (FTMT)、二价金属转运蛋白 1 (DMT1)、铁转运蛋白 1 (FPN1)、铁调节蛋白(IRP1 和 IRP2)和转铁蛋白受体 1 (TFR1)。值得注意的是,衰老的卵母细胞表现出铁蛋白自噬和线粒体自噬增强,并且胞质 Fe2+ 增加、脂质过氧化升高、线粒体功能障碍和溶酶体活性增强。此外,p53、p21、p16 和微管相关蛋白 tau (Tau) 的卵巢表达也被发现上调。这些改变可以通过体外给予 2 个月大小鼠的卵母细胞 Fe2+ 来进行表型复制,但可以通过去铁胺 (DFO) 得到缓解。 DFO的体内应用改善了12月龄小鼠的卵巢铁代谢和氧化还原状态,并纠正了胞浆Fe、铁蛋白自噬和线粒体自噬的改变以及卵母细胞的相关退行性变化。因此,总体而言,DFO 延缓了卵巢储备的下降,并显着增加了超排卵母细胞的数量,同时减少了碎片和非整倍体。 总之,我们的研究结果表明,与衰老相关的卵巢铁稳态紊乱导致ROS产生过多,而铁螯合可以改善卵巢氧化还原状态,并有效延缓衰老小鼠卵巢储备和卵母细胞质量的下降。这些数据提出了一种保护老年女性卵巢储备功能的新干预策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号