当前位置:
X-MOL 学术
›
Adv. Drug Deliver. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Long-acting transdermal drug delivery formulations: Current developments and innovative pharmaceutical approaches
Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2024-04-30 , DOI: 10.1016/j.addr.2024.115326 Tanvi Karve 1 , Amruta Dandekar 1 , Vivek Agrahari 2 , M Melissa Peet 2 , Ajay K Banga 1 , Gustavo F Doncel 2
Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2024-04-30 , DOI: 10.1016/j.addr.2024.115326 Tanvi Karve 1 , Amruta Dandekar 1 , Vivek Agrahari 2 , M Melissa Peet 2 , Ajay K Banga 1 , Gustavo F Doncel 2
Affiliation
|
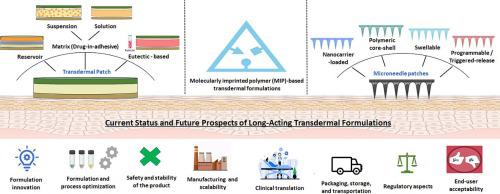
Transdermal administration remains an active research and development area as an alternative route for long-acting drug delivery. It avoids major drawbacks of conventional oral (gastrointestinal side effects, low drug bioavailability, and need for multiple dosing) or parenteral routes (invasiveness, pain, and psychological stress and bio-hazardous waste generated from needles), thereby increasing patient appeal and compliance. This review focuses on the current state of long-acting transdermal drug delivery, including adhesive patches, microneedles, and molecularly imprinted polymeric systems. Each subsection describes an approach including key considerations in formulation development, design, and process parameters with schematics. An overview of commercially available conventional (adhesive) patches for long-acting drug delivery (longer than 24 h), the reservoir- and matrix-type systems under preclinical evaluation, as well as the advanced transdermal formulations, such as the core-shell, nanoformulations-incorporated and stimuli-responsive microneedles, and 3D-printed and molecularly imprinted polymers that are in development, is also provided. Finally, we elaborated on translational aspects, challenges in patch formulation development, and future directions for the clinical advancement of new long-acting transdermal products.
中文翻译:

长效透皮给药制剂:当前发展和创新制药方法
作为长效药物输送的替代途径,透皮给药仍然是一个活跃的研究和开发领域。它避免了传统口服途径(胃肠道副作用、药物生物利用度低、需要多次给药)或肠胃外途径(侵入性、疼痛、心理压力以及针头产生的生物危险废物)的主要缺点,从而提高了患者的吸引力和依从性。本综述重点关注长效透皮给药的现状,包括贴剂、微针和分子印迹聚合物系统。每个小节都描述了一种方法,包括配方开发、设计和工艺参数中的关键考虑因素以及示意图。概述市售的长效药物输送(超过 24 小时)的常规(粘合)贴剂、临床前评估的储库型和基质型系统,以及先进的透皮制剂,如核-壳、还提供了结合纳米制剂和刺激响应的微针,以及正在开发的 3D 打印和分子印迹聚合物。最后,我们详细阐述了转化方面、贴剂配方开发的挑战以及新型长效透皮产品临床进展的未来方向。
更新日期:2024-04-30
中文翻译:

长效透皮给药制剂:当前发展和创新制药方法
作为长效药物输送的替代途径,透皮给药仍然是一个活跃的研究和开发领域。它避免了传统口服途径(胃肠道副作用、药物生物利用度低、需要多次给药)或肠胃外途径(侵入性、疼痛、心理压力以及针头产生的生物危险废物)的主要缺点,从而提高了患者的吸引力和依从性。本综述重点关注长效透皮给药的现状,包括贴剂、微针和分子印迹聚合物系统。每个小节都描述了一种方法,包括配方开发、设计和工艺参数中的关键考虑因素以及示意图。概述市售的长效药物输送(超过 24 小时)的常规(粘合)贴剂、临床前评估的储库型和基质型系统,以及先进的透皮制剂,如核-壳、还提供了结合纳米制剂和刺激响应的微针,以及正在开发的 3D 打印和分子印迹聚合物。最后,我们详细阐述了转化方面、贴剂配方开发的挑战以及新型长效透皮产品临床进展的未来方向。































 京公网安备 11010802027423号
京公网安备 11010802027423号