当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reduction of dinitrogen to ammonium through a magnesium-based electrochemical process at close-to-ambient temperature
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-05-23 , DOI: 10.1039/d4ee01090f Melinda Krebsz 1 , Rebecca Y. Hodgetts 1 , Sam Johnston 1 , Cuong K. Nguyen 1 , Yvonne Hora 2 , Douglas R. MacFarlane 1 , Alexandr N. Simonov 1
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-05-23 , DOI: 10.1039/d4ee01090f Melinda Krebsz 1 , Rebecca Y. Hodgetts 1 , Sam Johnston 1 , Cuong K. Nguyen 1 , Yvonne Hora 2 , Douglas R. MacFarlane 1 , Alexandr N. Simonov 1
Affiliation
|
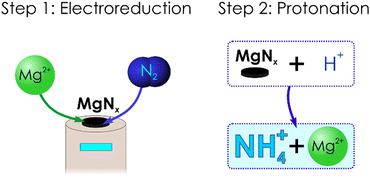
The Li-mediated nitrogen reduction enables practical synthesis of ammonia, but its energy efficiency is limited by the Li0/+ redox potential. To improve on this, a mediator with a more positive potential, like Mg0/2+, is required. Herein, we demonstrate that electrodeposited Mg0 reacts with N2 at a close-to-ambient temperature, and the resulting material can be converted into NH4+. This two-step process can produce ammonium with faradaic efficiency of up to 7% and yield rate up to 66 nmol s−1 cm−2.
中文翻译:

在接近环境温度下通过镁基电化学过程将氮还原为铵
锂介导的氮还原使得氨的实际合成成为可能,但其能量效率受到锂 0/+ 氧化还原电位的限制。为了改善这一点,需要具有更正电势的介体,例如 Mg 0/2+ 。在此,我们证明电沉积的 Mg 0 在接近环境温度下与 N 2 发生反应,所得材料可以转化为 NH 4 < b5> 。该两步法可生产铵,法拉第效率高达 7%,产率高达 66 nmol s −1 cm −2 。
更新日期:2024-05-23
中文翻译:

在接近环境温度下通过镁基电化学过程将氮还原为铵
锂介导的氮还原使得氨的实际合成成为可能,但其能量效率受到锂 0/+ 氧化还原电位的限制。为了改善这一点,需要具有更正电势的介体,例如 Mg 0/2+ 。在此,我们证明电沉积的 Mg 0 在接近环境温度下与 N 2 发生反应,所得材料可以转化为 NH 4 < b5> 。该两步法可生产铵,法拉第效率高达 7%,产率高达 66 nmol s −1 cm −2 。






































 京公网安备 11010802027423号
京公网安备 11010802027423号