当前位置:
X-MOL 学术
›
Nano Today
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cytomembrane-targeted photodynamic priming triggers extracellular vesicle storm for deep penetration and complete destruction of bladder cancer
Nano Today ( IF 13.2 ) Pub Date : 2024-05-17 , DOI: 10.1016/j.nantod.2024.102311
Xia Wang , Shipeng Ning , Wenhui Tao , Kaiyuan Wang , Juanjuan Li , Linghong Huang , Songtao Dong , Zhijin Fan , Judun Zheng , Yang Li , Bin Yang , Zhonggui He , Jin Sun , Xiaoyuan Chen , Hongxing Liu
Nano Today ( IF 13.2 ) Pub Date : 2024-05-17 , DOI: 10.1016/j.nantod.2024.102311
Xia Wang , Shipeng Ning , Wenhui Tao , Kaiyuan Wang , Juanjuan Li , Linghong Huang , Songtao Dong , Zhijin Fan , Judun Zheng , Yang Li , Bin Yang , Zhonggui He , Jin Sun , Xiaoyuan Chen , Hongxing Liu
|
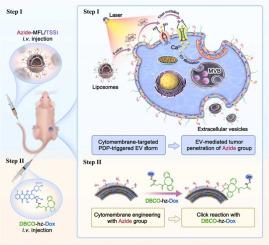
The overexpressed extracellular matrix and elevated interstitial fluid pressure in tumor extremely restricts deep infiltration of nanomedicines, which makes poor penetration an unsolved problem for potent antitumor drug delivery. It has been demonstrated that sub-cytotoxic photodynamic priming (PDP) could disrupt and permeabilize extracellular matrix to promote the tumor penetration of nanotherapeutics. However, given high interstitial fluid pressure, the efficacy is always limited owing to the passive diffusion of huge nanoparticles. Here, we, for the first time, find that cytomembrane-targeted PDP can effectively induce extracellular vesicle (EV) storm to enable intercellular transport of azide-containing ligands for active spread throughout the whole tumor, and thereafter destroy the entire tumor via bioorthogonal reaction. The membrane fusogenic liposome incorporating azide lipids is designed to encapsulate aggregation-induced emission (AIE)-active luminogen (AIEgen)-based photosensitizer TSSI. Following tumor accumulation, both azide ligands and TSSI could be transported to the cancer cell membrane and further packaged into the membrane of EVs. Under laser irradiation, PDP activates calcium channels on the cytomembrane via reactive oxygen species (ROS)-dependent thiol oxidation to boost cytosolic calcium influx, which is the main inducer of EVs. Endogenous EVs generated by outer layer cells provoke the intercellular delivery of azide ligands and TSSI into deep regions layer-by-layer. Then, the dibenzocyclooctyne (DBCO)-doxorubicin conjugate is intravenously administrated, which can effectively, selectively, and irreversibly react with azide lipids on the cell surface via bioorthogonal chemistry, allowing facilitated drug penetration and excellent therapeutic outcomes.
中文翻译:

细胞膜靶向光动力启动触发细胞外囊泡风暴,实现膀胱癌的深度渗透和完全破坏
肿瘤中过度表达的细胞外基质和升高的间质液压力极大地限制了纳米药物的深层渗透,这使得渗透性差成为有效抗肿瘤药物递送的未解决的问题。已证明亚细胞毒性光动力引发(PDP)可以破坏细胞外基质并使其通透,从而促进纳米治疗药物的肿瘤渗透。然而,由于间质液压力较高,由于巨大纳米颗粒的被动扩散,其功效始终受到限制。在这里,我们首次发现细胞膜靶向的PDP可以有效诱导细胞外囊泡(EV)风暴,使含叠氮配体能够在细胞间运输,从而主动扩散到整个肿瘤,然后通过生物正交反应破坏整个肿瘤。掺有叠氮脂质的膜融合脂质体旨在封装基于聚集诱导发射 (AIE) 活性发光体 (AIEgen) 的光敏剂 TSSI。肿瘤积累后,叠氮配体和 TSSI 都可以被转运到癌细胞膜并进一步包装到 EV 膜中。在激光照射下,PDP 通过活性氧 (ROS) 依赖性硫醇氧化激活细胞膜上的钙通道,以促进细胞质钙内流,这是 EV 的主要诱导剂。外层细胞产生的内源性 EV 刺激细胞间将叠氮化物配体和 TSSI 逐层输送到深层区域。 然后,静脉注射二苯并环辛炔(DBCO)-阿霉素缀合物,它可以通过生物正交化学有效地、选择性地、不可逆地与细胞表面的叠氮脂质发生反应,从而促进药物渗透并获得良好的治疗效果。
更新日期:2024-05-17
中文翻译:

细胞膜靶向光动力启动触发细胞外囊泡风暴,实现膀胱癌的深度渗透和完全破坏
肿瘤中过度表达的细胞外基质和升高的间质液压力极大地限制了纳米药物的深层渗透,这使得渗透性差成为有效抗肿瘤药物递送的未解决的问题。已证明亚细胞毒性光动力引发(PDP)可以破坏细胞外基质并使其通透,从而促进纳米治疗药物的肿瘤渗透。然而,由于间质液压力较高,由于巨大纳米颗粒的被动扩散,其功效始终受到限制。在这里,我们首次发现细胞膜靶向的PDP可以有效诱导细胞外囊泡(EV)风暴,使含叠氮配体能够在细胞间运输,从而主动扩散到整个肿瘤,然后通过生物正交反应破坏整个肿瘤。掺有叠氮脂质的膜融合脂质体旨在封装基于聚集诱导发射 (AIE) 活性发光体 (AIEgen) 的光敏剂 TSSI。肿瘤积累后,叠氮配体和 TSSI 都可以被转运到癌细胞膜并进一步包装到 EV 膜中。在激光照射下,PDP 通过活性氧 (ROS) 依赖性硫醇氧化激活细胞膜上的钙通道,以促进细胞质钙内流,这是 EV 的主要诱导剂。外层细胞产生的内源性 EV 刺激细胞间将叠氮化物配体和 TSSI 逐层输送到深层区域。 然后,静脉注射二苯并环辛炔(DBCO)-阿霉素缀合物,它可以通过生物正交化学有效地、选择性地、不可逆地与细胞表面的叠氮脂质发生反应,从而促进药物渗透并获得良好的治疗效果。

































 京公网安备 11010802027423号
京公网安备 11010802027423号