当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Weakly solvating aqueous-based electrolyte facilitated by a soft co-solvent for extreme temperature operations of zinc-ion batteries
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-05-21 , DOI: 10.1039/d4ee00942h Ruizhi Zhang 1, 2, 3 , Wei Kong Pang 2 , Jitraporn (Pimm) Vongsvivut 4 , Jodie A. Yuwono 1 , Guanjie Li 1 , Yanqiu Lyu 1 , Yameng Fan 2 , Yunlong Zhao 5 , Shilin Zhang 1 , Jianfeng Mao 1 , Qiong Cai 3 , Sailin Liu 1 , Zaiping Guo 1
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-05-21 , DOI: 10.1039/d4ee00942h Ruizhi Zhang 1, 2, 3 , Wei Kong Pang 2 , Jitraporn (Pimm) Vongsvivut 4 , Jodie A. Yuwono 1 , Guanjie Li 1 , Yanqiu Lyu 1 , Yameng Fan 2 , Yunlong Zhao 5 , Shilin Zhang 1 , Jianfeng Mao 1 , Qiong Cai 3 , Sailin Liu 1 , Zaiping Guo 1
Affiliation
|
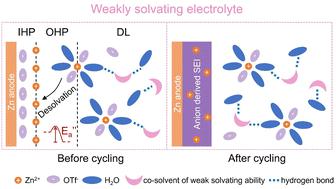
The aqueous zinc-ion battery (AZIB) is a promising option for grid-scale energy storage, but it faces challenges from parasitic water-related reactions and limited operational temperature range. Replacing H2O molecules in the solvation sheath of Zn2+ with strongly solvating co-solvents can effectively suppress water-related side reactions. However, the excessive Zn2+–co-solvent interaction can cause a large activation energy of desolvation (Ea) and the decomposition of the co-solvent may introduce non-ionic conductive solid electrolyte interphase (SEI) species. Hence, we propose a weakly solvating electrolyte that adopts diethylene glycol dimethyl ether (G2) as a soft co-solvent. The G2 has a moderate Gutmann donor number (19 kcal mol−1) and a low dielectric constant (7.4), which reduces the presence of water in the solvation sheath and enhances Zn2+–anion interaction. This electrolyte achieves an optimal Ea and a robust anion-derived SEI (ZnS–ZnSO3–ZnF2) on the zinc anode, allowing highly reversible Zn plating/stripping for over 7500 hours. The strong G2–H2O interaction enables G2 to bind free H2O and reconstruct the hydrogen bond network, which prevents water decomposition and widens the electrolyte's operational temperature range (−60 °C to 60 °C). The Zn//KV12O30−y·nH2O (KVOH) full battery delivers a high-capacity retention of 91.2% following 8000 cycles at 5.0 A g−1 at room temperature. It also achieves capacity retention of 82.9% over 4000 cycles (0.1 A g−1) at −45 °C and 86.5% for 1200 cycles (5.0 A g−1) at 60 °C, respectively. This work optimizes interface chemistry and temperature adaptability of AZIBs, offering guidance for designing weakly solvating aqueous-based electrolytes towards practical application.
中文翻译:

软助溶剂促进弱溶剂化水基电解质,用于锌离子电池的极端温度操作
水系锌离子电池(AZIB)是电网规模储能的一种有前途的选择,但它面临着寄生水相关反应和有限的工作温度范围的挑战。用强溶剂化共溶剂取代Zn 2+ 溶剂化层中的H 2 O分子可以有效抑制与水相关的副反应。然而,过量的Zn 2+ - 共溶剂相互作用会导致去溶剂化活化能(E a )较大,并且共溶剂的分解可能会引入非离子导电固体电解质间相(SEI)种类。因此,我们提出了一种弱溶剂化电解质,采用二甘醇二甲醚(G2)作为软助溶剂。 G2 具有适中的古特曼供体数(19 kcal mol −1 )和低介电常数(7.4),可减少溶剂化鞘中水的存在并增强 Zn 2+ –阴离子相互作用。该电解质在锌阳极上实现了最佳 E a 和强大的阴离子衍生 SEI (ZnS–ZnSO 3 –ZnF 2 ),从而实现高度可逆的 Zn电镀/剥离超过 7500 小时。强烈的G2-H 2 O相互作用使G2能够结合游离的H 2 O并重建氢键网络,从而防止水分解并扩大电解质的工作温度范围(−60° C 至 60°C)。 Zn//KV 12 O 30−y ·nH 2 O (KVOH) 全电池在 5.0 A 电流下循环 8000 次后,容量保持率高达 91.2% g −1 在室温下。它还在 -45 °C 下 4000 次循环 (0.1 A g −1 ) 中实现了 82.9% 的容量保持率,在 60 °C 下 1200 次循环 (5.0 A g −1 ) 中容量保持率达到 86.5% , 分别。 这项工作优化了 AZIB 的界面化学和温度适应性,为设计弱溶剂化水基电解质以实现实际应用提供指导。
更新日期:2024-05-21
中文翻译:

软助溶剂促进弱溶剂化水基电解质,用于锌离子电池的极端温度操作
水系锌离子电池(AZIB)是电网规模储能的一种有前途的选择,但它面临着寄生水相关反应和有限的工作温度范围的挑战。用强溶剂化共溶剂取代Zn 2+ 溶剂化层中的H 2 O分子可以有效抑制与水相关的副反应。然而,过量的Zn 2+ - 共溶剂相互作用会导致去溶剂化活化能(E a )较大,并且共溶剂的分解可能会引入非离子导电固体电解质间相(SEI)种类。因此,我们提出了一种弱溶剂化电解质,采用二甘醇二甲醚(G2)作为软助溶剂。 G2 具有适中的古特曼供体数(19 kcal mol −1 )和低介电常数(7.4),可减少溶剂化鞘中水的存在并增强 Zn 2+ –阴离子相互作用。该电解质在锌阳极上实现了最佳 E a 和强大的阴离子衍生 SEI (ZnS–ZnSO 3 –ZnF 2 ),从而实现高度可逆的 Zn电镀/剥离超过 7500 小时。强烈的G2-H 2 O相互作用使G2能够结合游离的H 2 O并重建氢键网络,从而防止水分解并扩大电解质的工作温度范围(−60° C 至 60°C)。 Zn//KV 12 O 30−y ·nH 2 O (KVOH) 全电池在 5.0 A 电流下循环 8000 次后,容量保持率高达 91.2% g −1 在室温下。它还在 -45 °C 下 4000 次循环 (0.1 A g −1 ) 中实现了 82.9% 的容量保持率,在 60 °C 下 1200 次循环 (5.0 A g −1 ) 中容量保持率达到 86.5% , 分别。 这项工作优化了 AZIB 的界面化学和温度适应性,为设计弱溶剂化水基电解质以实现实际应用提供指导。

































 京公网安备 11010802027423号
京公网安备 11010802027423号