当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Umpolung Flow Chemistry for the Synthesis of a 3-Oxo-3H-spiro[benzofuran-2,4′-piperidine] Building Block
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-05-20 , DOI: 10.1021/acs.joc.4c00337 Matthew M Pompeo 1 , Sean M Kelly 1 , Frédéric St-Jean 1 , Thomas M Bass 1 , Derek M Dalton 1 , Daniel Zell 1 , Chong Han 1 , Lauren E Sirois 1 , Francis Gosselin 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-05-20 , DOI: 10.1021/acs.joc.4c00337 Matthew M Pompeo 1 , Sean M Kelly 1 , Frédéric St-Jean 1 , Thomas M Bass 1 , Derek M Dalton 1 , Daniel Zell 1 , Chong Han 1 , Lauren E Sirois 1 , Francis Gosselin 1
Affiliation
|
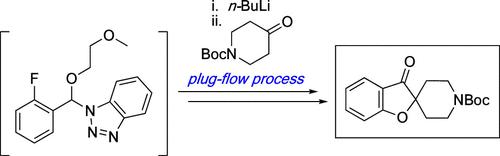
An efficient and scalable route to tert-butyl 3-oxo-3H-spiro[benzofuran-2,4′-piperidine]-1′-carboxylate, a central prochiral intermediate in the synthesis of SHP2 inhibitor GDC-1971 (migoprotafib), was achieved. Preparation of the title compound from readily available 2-fluorobenzaldehyde included formation of a modified Katritzky benzotriazole hemiaminal, which, upon deprotonation by n-butyllithium, participated in umpolung reactivity via 1,2-addition to tert-butyl 4-oxopiperidine-1-carboxylate (N-Boc-4-piperidone). Most notably, this reaction was developed as a robust plug-flow process that could be executed on multiple kilograms without the need for pilot-scale reaction vessels operating at low cryogenic temperatures. Treatment of the resulting tetrahedral intermediate with oxalic acid resulted in collapse to the corresponding 4-(2-fluorobenzoyl)-4-hydroxypiperidine, which was isolated as a solid via crystallization. The synthesis concluded with an optimized intramolecular SNAr reaction and final crystallization to generate tert-butyl 3-oxo-3H-spiro[benzofuran-2,4′-piperidine]-1′-carboxylate as a stable, high-quality intermediate suitable for further functionalization toward GDC-1971.
中文翻译:

用于合成 3-Oxo-3H-spiro[苯并呋喃-2,4′-哌啶]结构单元的 Umpolung 流动化学
一种高效且可扩展的路线,用于合成3-oxo-3 H-螺环[苯并呋喃-2,4'-哌啶]-1'-甲酸叔丁酯,这是合成 SHP2 抑制剂GDC-1971 ( migoprotafib ) 的中心前手性中间体,已实现。从容易获得的 2-氟苯甲醛制备标题化合物包括形成改性的 Katritzky 苯并三唑半缩醛,其在被正丁基锂去质子化后,通过 1,2-加成到 4-氧代哌啶-1-甲酸叔丁酯参与反极性反应。 ( N -Boc-4-哌啶酮)。最值得注意的是,该反应被开发为一种强大的活塞流工艺,可以在数公斤上执行,而不需要在低温下运行的中试规模反应容器。用草酸处理所得四面体中间体导致塌陷成相应的4-(2-氟苯甲酰基)-4-羟基哌啶,其通过结晶分离为固体。合成以优化的分子内 S N Ar 反应和最终结晶结束,生成稳定的高质量中间体3-oxo-3 H-螺环[苯并呋喃-2,4'-哌啶]-1'-甲酸叔丁酯适合针对GDC-1971进行进一步功能化。
更新日期:2024-05-20
中文翻译:

用于合成 3-Oxo-3H-spiro[苯并呋喃-2,4′-哌啶]结构单元的 Umpolung 流动化学
一种高效且可扩展的路线,用于合成3-oxo-3 H-螺环[苯并呋喃-2,4'-哌啶]-1'-甲酸叔丁酯,这是合成 SHP2 抑制剂GDC-1971 ( migoprotafib ) 的中心前手性中间体,已实现。从容易获得的 2-氟苯甲醛制备标题化合物包括形成改性的 Katritzky 苯并三唑半缩醛,其在被正丁基锂去质子化后,通过 1,2-加成到 4-氧代哌啶-1-甲酸叔丁酯参与反极性反应。 ( N -Boc-4-哌啶酮)。最值得注意的是,该反应被开发为一种强大的活塞流工艺,可以在数公斤上执行,而不需要在低温下运行的中试规模反应容器。用草酸处理所得四面体中间体导致塌陷成相应的4-(2-氟苯甲酰基)-4-羟基哌啶,其通过结晶分离为固体。合成以优化的分子内 S N Ar 反应和最终结晶结束,生成稳定的高质量中间体3-oxo-3 H-螺环[苯并呋喃-2,4'-哌啶]-1'-甲酸叔丁酯适合针对GDC-1971进行进一步功能化。































 京公网安备 11010802027423号
京公网安备 11010802027423号