Cell Metabolism ( IF 27.7 ) Pub Date : 2024-05-20 , DOI: 10.1016/j.cmet.2024.04.018 Fei Peng 1 , Jinxin Lu 1 , Keyu Su 2 , Xinyu Liu 3 , Huandong Luo 1 , Bin He 1 , Cenxin Wang 1 , Xiaoyu Zhang 1 , Fan An 1 , Dekang Lv 1 , Yuanyuan Luo 4 , Qitong Su 1 , Tonghui Jiang 1 , Ziqian Deng 1 , Bin He 5 , Lingzhi Xu 6 , Tao Guo 7 , Jin Xiang 5 , Chundong Gu 7 , Ling Wang 8 , Guowang Xu 3 , Ying Xu 9 , Mindian Li 10 , Keith W Kelley 11 , Bai Cui 2 , Quentin Liu 2
|
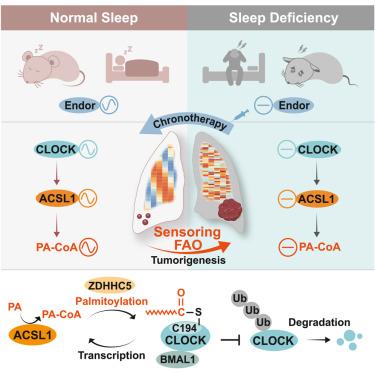
Circadian disruption predicts poor cancer prognosis, yet how circadian disruption is sensed in sleep-deficiency (SD)-enhanced tumorigenesis remains obscure. Here, we show fatty acid oxidation (FAO) as a circadian sensor relaying from clock disruption to oncogenic metabolic signal in SD-enhanced lung tumorigenesis. Both unbiased transcriptomic and metabolomic analyses reveal that FAO senses SD-induced circadian disruption, as illustrated by continuously increased palmitoyl-coenzyme A (PA-CoA) catalyzed by long-chain fatty acyl-CoA synthetase 1 (ACSL1). Mechanistically, SD-dysregulated CLOCK hypertransactivates ACSL1 to produce PA-CoA, which facilitates CLOCK-Cys194 S-palmitoylation in a ZDHHC5-dependent manner. This positive transcription-palmitoylation feedback loop prevents ubiquitin-proteasomal degradation of CLOCK, causing FAO-sensed circadian disruption to maintain SD-enhanced cancer stemness. Intriguingly, timed β-endorphin resets rhythmic Clock and Acsl1 expression to alleviate SD-enhanced tumorigenesis. Sleep quality and serum β-endorphin are negatively associated with both cancer development and CLOCK/ACSL1 expression in patients with cancer, suggesting dawn-supplemented β-endorphin as a potential chronotherapeutic strategy for SD-related cancer.
中文翻译:

致癌脂肪酸氧化感知睡眠不足增强肿瘤发生中的昼夜节律破坏
昼夜节律紊乱预示着癌症预后不佳,但如何在睡眠不足(SD)增强的肿瘤发生中感知昼夜节律紊乱仍然不清楚。在这里,我们展示了脂肪酸氧化 (FAO) 作为一种昼夜节律传感器,在 SD 增强的肺部肿瘤发生中从时钟中断传递到致癌代谢信号。无偏见的转录组学和代谢组学分析均表明,FAO 感受到了 SD 诱导的昼夜节律紊乱,如长链脂肪酰辅酶 A 合成酶 1 (ACSL1) 催化的棕榈酰辅酶 A (PA-CoA) 持续增加所示。从机制上讲,SD 失调的 CLOCK 过度激活 ACSL1 产生 PA-CoA,从而以 ZDHHC5 依赖性方式促进 CLOCK-Cys194 S-棕榈酰化。这种正转录-棕榈酰化反馈环路可防止 CLOCK 的泛素-蛋白酶体降解,从而导致 FAO 感知的昼夜节律紊乱,从而维持 SD 增强的癌症干细胞性。有趣的是,定时 β-内啡肽重置节律时钟和Acsl1表达,以减轻 SD 增强的肿瘤发生。睡眠质量和血清 β-内啡肽与癌症患者的癌症发展和 CLOCK/ACSL1 表达呈负相关,这表明补充 β-内啡肽可作为 SD 相关癌症的潜在时间治疗策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号