当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Access to Main-Chain Photoswitching Polymers via Hydroxyl-yne Click Polymerization
ACS Macro Letters ( IF 5.1 ) Pub Date : 2024-05-16 , DOI: 10.1021/acsmacrolett.4c00216 Linh Duy Thai 1, 2, 3 , Jochen A. Kammerer 1, 2 , Patrick Théato 4, 5 , Hatice Mutlu 6 , Christopher Barner-Kowollik 1, 2, 3
ACS Macro Letters ( IF 5.1 ) Pub Date : 2024-05-16 , DOI: 10.1021/acsmacrolett.4c00216 Linh Duy Thai 1, 2, 3 , Jochen A. Kammerer 1, 2 , Patrick Théato 4, 5 , Hatice Mutlu 6 , Christopher Barner-Kowollik 1, 2, 3
Affiliation
|
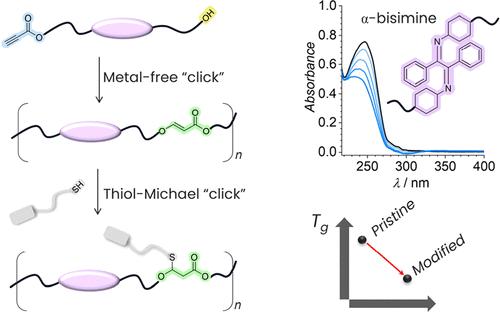
Main-chain stimuli-responsive polymers synthesized via polymerization techniques that do not rely on metal-based catalysis are highly desirable for economic reasons and to avoid metal–polymer interactions. Herein, we introduce a metal-free head-to-tail organobase-catalyzed hydroxyl-yne click polymerization of an AB-type monomer to realize photoswitchable polymers featuring α-bismines as main-chain repeating units. The prepared main-chain α-bisimine-based polymers show excellent photoswitching in solution. We further post-functionalize the obtained polymers with various thiol compounds via thiol-Michael reactions to significantly lower the glass transition temperature (Tg), likely to be beneficial for the photoswitching process in the solid state. Thus, the herein introduced polymerization technique not only provides metal-free access to main-chain stimuli-responsive polymers, but also allows for the flexible post-modification of the obtained polymers to generate advanced macromolecular architectures with tunable properties.
中文翻译:

通过羟基炔点击聚合获得主链光开关聚合物
出于经济原因和避免金属-聚合物相互作用,通过不依赖金属催化的聚合技术合成的主链刺激响应聚合物是非常理想的。在此,我们引入了AB型单体的无金属头尾有机碱催化的羟基-炔点击聚合,以实现以α-双胺作为主链重复单元的光可切换聚合物。所制备的主链α-双亚胺基聚合物在溶液中表现出优异的光开关性能。我们进一步通过硫醇-迈克尔反应用各种硫醇化合物对所得聚合物进行后官能化,以显着降低玻璃化转变温度(T g ),这可能有利于固态光开关过程。因此,本文引入的聚合技术不仅提供了主链刺激响应聚合物的无金属途径,而且还允许对所获得的聚合物进行灵活的后修饰,以产生具有可调特性的先进大分子结构。
更新日期:2024-05-16
中文翻译:

通过羟基炔点击聚合获得主链光开关聚合物
出于经济原因和避免金属-聚合物相互作用,通过不依赖金属催化的聚合技术合成的主链刺激响应聚合物是非常理想的。在此,我们引入了AB型单体的无金属头尾有机碱催化的羟基-炔点击聚合,以实现以α-双胺作为主链重复单元的光可切换聚合物。所制备的主链α-双亚胺基聚合物在溶液中表现出优异的光开关性能。我们进一步通过硫醇-迈克尔反应用各种硫醇化合物对所得聚合物进行后官能化,以显着降低玻璃化转变温度(T g ),这可能有利于固态光开关过程。因此,本文引入的聚合技术不仅提供了主链刺激响应聚合物的无金属途径,而且还允许对所获得的聚合物进行灵活的后修饰,以产生具有可调特性的先进大分子结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号