当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Flow Chemistry and Continuous Processing: More Mainstream than Ever!
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-05-17 , DOI: 10.1021/acs.oprd.3c00483 Kevin P. Cole 1 , Jonathan N. Jaworski 2 , C. Oliver Kappe 3 , Shu Kobayashi 4 , Anita R. Maguire 5 , Anne O’Kearney-McMullan 6 , Jaan A. Pesti 7
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-05-17 , DOI: 10.1021/acs.oprd.3c00483 Kevin P. Cole 1 , Jonathan N. Jaworski 2 , C. Oliver Kappe 3 , Shu Kobayashi 4 , Anita R. Maguire 5 , Anne O’Kearney-McMullan 6 , Jaan A. Pesti 7
Affiliation
|
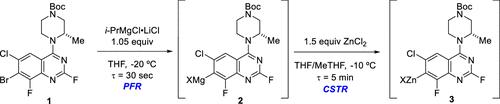
This article is part of the Flow Chemistry Enabling Efficient Synthesis 2024 special issue. We are pleased to present the fifth OPR&D special issue focused on continuous processing and hope that it pays homage to the previous editions by inspiring further momentum in this exciting field! It is safe to say that continuous manufacturing (CM) can now be viewed as a mainstream technology that is widely implemented across fine chemicals, agrochemicals, and pharmaceuticals. It is now commonplace to meet (under)graduate students or postdocs who have experience performing reactions in flow, and the importance of academic research in flow chemistry has never been greater. This is key to supplying the workforce with fresh talent trained in CM, but companies must continue to invest in the training and education of their existing staff to keep a critical mass of CM know-how. While there is no arguing that industrial chemists have often provided leadership regarding a multitude of continuous flow concepts, they frequently rely on academia to pioneer new reaction or equipment types and to “lure” them into applying the new methods to industrial problems. With that can come important academic–industrial collaborations, which in addition to training students in CM has served to expedite much of the cutting-edge research coming out of universities. Of the pharmaceutical companies that possess their own manufacturing capabilities, many if not most have recently implemented divisions or personnel that specialize in flow processes. While this fact is encouraging, it may hide missed opportunities to push the limitations of CM. Many agrochemical and pharmaceutical manufacturers outsource the preparation of advanced intermediates and conduct only the final key steps, which are often less chemically complex, in-house. There are tremendous opportunities for CM upstream from the final sequence, where many of the most difficult chemical transformations are conducted, often by contract manufacturing organizations (CMOs). It is obvious that CM capabilities are now increasing among CMOs in our industries. Many contract manufacturers in fine chemicals or pharmaceuticals offer various continuous processing capabilities. These range from simple plug-flow reactors (PFRs) or continuous stirred tank reactors (CSTRs) to more sophisticated equipment modalities such as photochemical or packed bed hydrogenation reactors and even the ability to conduct separations such as extraction or crystallization in continuous flow. What is perhaps most encouraging is that some CMOs now possess true competence in these areas and can develop and implement certain CM processes with minimal input from the customer. While this is not true for every case, none of it was true a decade ago when most CMOs had either not begun to implement CM or were only beginning to move in this direction; an IQ survey from ca. 2018 discusses this topic. (1) What caused such a rapid change in the level of adoption of CM technologies among CMOs? The answer is undoubtedly complex, but it certainly includes the manufacturers realizing the benefits of these technologies and increasing CM demand from their clients. The most important benefits of flow have not changed, but CMOs appear to have realized that they must offer CM capabilities or lose clients to competitors. We would be remiss as well to ignore the changes that have occurred in the regulatory space concerning the growing adaptation of CM. With the finalization of ICH Q13, (2) a framework for how CM processes can be developed and communicated to regulators is in place, and the fear of “how will regulators treat my CM submission?” is reduced. Looking to the future, the merits of conducting so-called “end-to-end continuous manufacturing” can be argued (and are!). End-to-end processes, and even merely linked and simultaneously operating CM processes (e.g., a PFR and a linked separation unit operation) are still relatively uncommon in our industries. We often select a batch–flow hybrid model, where a standalone flow unit operation (normally a reaction) is conducted and the subsequent workup is performed in batch mode. When adjacent flow unit operations are linked, the complexity and challenge of conducting these operations greatly increases, especially if the processes are conducted under rigorous quality constraints such as current Good Manufacturing Practices (cGMPs). It is important to note, however, that there is tremendous unrealized potential in continuous separation unit operations. Separations that are run continuously such as countercurrent extraction, thin-film evaporation, or kinetically controlled crystallization can offer miraculous benefits over the batch analog in terms of yield, safety, impurity rejection, resource usage, etc. While many chemists and engineers are now trained to look for opportunities to implement flow reactions, we should also search for what beneficial flow separations might exist when designing and commercializing new syntheses. It is our belief that the next CM special issue will possess articles highlighting increased numbers of separations performed in flow. These capabilities will become as widespread as flow reaction unit operations. We sincerely thank all the authors who have contributed to this issue with their time and talent. There are many noteworthy articles in the 2024 special issue, but three particularly caught our attention. Many teams have described their development approaches toward covalent KRAS G12C inhibitors, (3) and this previously undruggable target has become popular throughout oncology drug development. (4) Many small-molecule inhibitors feature a densely functionalized biaryl ring system, some of which are atropisomeric. A team from Genentech and Roche (DOI: 10.1021/acs.oprd.3c00164) describe an excellent application of continuous flow technology in the Grignard exchange and subsequent transmetalation to the arylzinc reagent needed to forge the biaryl bond (Scheme 1). The continuous process enabled operation at higher temperature (−20 vs −70 °C), which was cited by the authors as being an important factor in the ability to scale the process to commercial volumes in available equipment. The continuous process was demonstrated at kilogram scale and gave a 72% isolated yield after the subsequent Negishi coupling. The formation of N–N bonds can be a powerful method for the synthesis of aromatic heterocycles. A team from TCG GreenChem Inc. (DOI: 10.1021/acs.oprd.3c00184) demonstrated the use of multistep continuous processing to form 2-cyanopyrrole from pyrrole (Scheme 2). In the next step, pyrrole N-amination is achieved in flow using in situ-generated chloramine. The previously reported base for the amination, sodium hydride, was replaced with safer t-BuOK in the flow process. Finally, the 1-aminopyrrole intermediate is treated with formamidine acetate in batch mode to afford desired pyrrolotriazine target. Several process safety improvements were noted as driving factors in this work, and the yield of the three-step sequence was 52% with high purity and assay. This work is impressive from the perspective that multiple simultaneous unit operations were connected, including separations, to enable multiple challenging chemical transformation to be achieved at once. Finally, an interesting example of continuous reactor design has been disclosed by Jiang and co-workers (DOI: 10.1021/acs.oprd.3c00328). A series of eight mini-CSTRs capable of vertical or horizontal flow was constructed with a built-in LED array, stirring, and cooling capabilities (Figure 1). This reactor design was demonstrated to tolerate use of a solid-supported catalyst, uranyl-doped glass wool, in addition to more standard catalysts. In the case of the glass-wool-supported catalyst, catalyst recycling of at least 12 cycles was demonstrated without loss of activity. The reactor was tested with a handful of photochemical applications, including oxidations and ketone carboxylation. This stands as an example of how improved reactor design can further enable specialized conditions for applications in CM. Figure 1. Eight CSTRs in series reactor for heterogeneous photocatalytic processes. Reproduced from 10.1021/acs.oprd.3c00328. Copyright 2023 American Chemical Society. We hope that you will enjoy this issue and take the time to read many of the articles in-depth. It was certainly a fun challenge to pull all these articles together for this issue, and we hope that the passion, dedication, and engagement of the authors is evident and infectious. Happy reading! This article references 4 other publications. For recent selected references related to KRAS inhibitor synthesis, see: This article has not yet been cited by other publications. Figure 1. Eight CSTRs in series reactor for heterogeneous photocatalytic processes. Reproduced from 10.1021/acs.oprd.3c00328. Copyright 2023 American Chemical Society. This article references 4 other publications. For recent selected references related to KRAS inhibitor synthesis, see:
中文翻译:

流动化学和连续处理:比以往更加主流!
本文是该文章的一部分流动化学实现高效合成 2024 专刊。我们很高兴推出第五期专注于连续加工的OPR&D特刊,并希望它能够在这个激动人心的领域激发进一步的动力,从而向前几版致敬!可以肯定地说,连续制造(CM)现在可以被视为一种主流技术,广泛应用于精细化学品、农用化学品和制药领域。现在,遇到具有在流动中进行反应的经验的(本科)研究生或博士后是很常见的,并且流动化学中的学术研究的重要性从未如此重要。这是为员工提供经过 CM 培训的新人才的关键,但公司必须继续投资于现有员工的培训和教育,以保持足够的 CM 专业知识。虽然毫无疑问,工业化学家经常在多种连续流概念方面发挥领导作用,但他们经常依靠学术界来开拓新的反应或设备类型,并“引诱”他们将新方法应用于工业问题。随之而来的是重要的学术与工业合作,除了培训 CM 学生之外,还有助于加快大学的许多前沿研究的发展。在拥有自己的制造能力的制药公司中,许多(如果不是大多数)最近都设立了专门从事流程工艺的部门或人员。虽然这一事实令人鼓舞,但它可能掩盖了错失的突破 CM 局限性的机会。许多农用化学品和药品制造商将高级中间体的制备外包,并仅在内部进行最后的关键步骤,这些步骤通常化学复杂度较低。最终序列上游的 CM 存在巨大的机会,其中许多最困难的化学转化通常由合同制造组织 (CMO) 进行。显然,我们行业的 CMO 的 CM 能力正在不断增强。许多精细化学品或制药合同制造商提供各种连续加工能力。这些范围从简单的活塞流反应器 (PFR) 或连续搅拌釜反应器 (CSTR) 到更复杂的设备模式,例如光化学或填充床氢化反应器,甚至能够进行分离,例如连续流中的萃取或结晶。也许最令人鼓舞的是,一些 CMO 现在在这些领域拥有真正的能力,并且可以在客户投入最少的情况下开发和实施某些 CM 流程。虽然并非所有情况都是如此,但十年前,当大多数 CMO 尚未开始实施 CM 或才开始朝这个方向发展时,情况并非如此。来自ca 的智商调查。 2018年讨论了这个话题。 (1) 是什么原因导致 CMO 中 CM 技术的采用水平发生如此迅速的变化?答案无疑是复杂的,但它肯定包括制造商认识到这些技术的好处以及客户日益增长的 CM 需求。 Flow 最重要的好处没有改变,但 CMO 似乎已经意识到,他们必须提供 CM 功能,否则就会失去客户给竞争对手。如果我们忽视了与 CM 日益适应相关的监管领域所发生的变化,那也是我们的失职。随着 ICH Q13 的最终确定,(2) 如何开发 CM 流程并向监管机构传达的框架已经到位,并且担心“监管机构将如何处理我的 CM 提交?”降低了。展望未来,进行所谓的“端到端连续制造”的优点是有争议的(而且确实如此!)。端到端流程,甚至只是链接并同时运行的 CM 流程(例如,PFR 和链接的分离单元操作)在我们的行业中仍然相对不常见。我们经常选择批量-流程混合模型,其中进行独立的流程单元操作(通常是反应),并以批量模式执行后续处理。当相邻的流动单元操作相互连接时,执行这些操作的复杂性和挑战会大大增加,特别是如果流程是在严格的质量限制(例如当前的良好生产规范(cGMP))下进行的。然而,值得注意的是,连续分离装置操作存在巨大的未实现潜力。连续运行的分离(例如逆流萃取、薄膜蒸发或动力学控制结晶)可以在产量、安全性、杂质去除、资源利用等方面比批量模拟提供神奇的好处。虽然许多化学家和工程师现在都接受过培训为了寻找实施流动反应的机会,我们还应该在设计和商业化新合成时寻找可能存在的有益流动分离。我们相信下一期 CM 特刊将包含强调流动中执行的分离数量增加的文章。这些功能将像流动反应单元操作一样广泛应用。我们衷心感谢所有为本期贡献时间和才华的作者。 2024 年特刊中有许多值得关注的文章,但其中三篇特别引起了我们的注意。许多团队已经描述了他们的共价 KRAS G12C 抑制剂的开发方法,(3),这种以前不可成药的靶点已在整个肿瘤药物开发中变得流行。 (4) 许多小分子抑制剂具有密集功能化的联芳环系统,其中一些是阻转异构体。来自基因泰克和罗氏的团队(DOI:10.1021/acs.oprd。3c00164)描述了连续流技术在格氏交换和随后的金属转移到形成联芳键所需的芳基锌试剂中的出色应用(方案 1)。连续工艺能够在更高的温度(-20 与 -70 °C)下运行,作者认为这是能够在现有设备中将该工艺扩展到商业规模的一个重要因素。该连续过程在公斤规模上进行了演示,并在随后的 Negishi 偶联后获得了 72% 的分离产率。 N-N键的形成是合成芳香杂环的有效方法。 TCG GreenChem Inc.(DOI:10.1021/acs.oprd.3c00184)的团队演示了如何使用多步连续加工从吡咯形成 2-氰基吡咯(方案 2)。下一步,吡咯N-胺化是使用原位产生的氯胺在流动中实现的。先前报道的胺化碱氢化钠在流动过程中被更安全的t -BuOK取代。最后,用甲脒乙酸酯以间歇模式处理1-氨基吡咯中间体,得到所需的吡咯并三嗪目标物。多项工艺安全改进被认为是这项工作的驱动因素,三步序列的产率为 52%,纯度和分析结果都很高。从多个同时进行的单元操作(包括分离)相连接的角度来看,这项工作令人印象深刻,从而能够同时实现多个具有挑战性的化学转化。最后,Jiang 及其同事公开了一个有趣的连续反应器设计示例(DOI:10.1021/acs.oprd.3c00328)。一系列八个能够垂直或水平流动的迷你 CSTR 构建有内置 LED 阵列、搅拌和冷却功能(图 1)。该反应器设计被证明除了更标准的催化剂外,还可以使用固体支撑的催化剂、铀酰掺杂的玻璃棉。在玻璃棉负载催化剂的情况下,催化剂可循环至少 12 个周期,且不会损失活性。该反应器经过了一些光化学应用的测试,包括氧化和酮羧化。这是改进的反应器设计如何进一步为 CM 应用提供特殊条件的一个例子。图 1. 串联反应器中的八个 CSTR 用于非均相光催化过程。转载自 10.1021/acs.oprd.3c00328。版权所有 2023 美国化学会。我们希望您会喜欢本期,并花时间深入阅读其中的许多文章。将本期所有这些文章整合在一起无疑是一个有趣的挑战,我们希望作者的热情、奉献和参与是显而易见的且具有感染力的。阅读愉快!本文参考了其他 4 篇出版物。有关 KRAS 抑制剂合成的最新精选参考文献,请参阅:本文尚未被其他出版物引用。图 1. 串联反应器中的八个 CSTR 用于非均相光催化过程。转载自 10.1021/acs.oprd.3c00328。版权所有 2023 美国化学会。本文参考了其他 4 篇出版物。有关 KRAS 抑制剂合成的最新精选参考文献,请参阅:
更新日期:2024-05-18
中文翻译:

流动化学和连续处理:比以往更加主流!
本文是该文章的一部分






































 京公网安备 11010802027423号
京公网安备 11010802027423号