当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interrupted Corey–Chaykovsky Reaction of Tethered Bis-Enones to Access 2,3-Epoxy-hexahydrofluoren-9-ones
Organic Letters ( IF 4.9 ) Pub Date : 2024-05-17 , DOI: 10.1021/acs.orglett.4c01550
Jay Prakash Maurya 1 , S S V Ramasastry 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-05-17 , DOI: 10.1021/acs.orglett.4c01550
Jay Prakash Maurya 1 , S S V Ramasastry 1
Affiliation
|
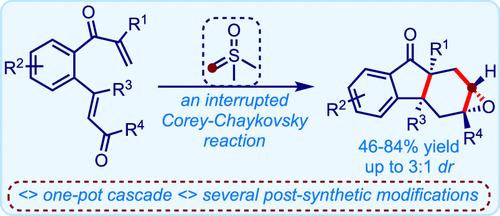
The Corey–Chaykovsky reaction is usually employed to synthesize cyclopropanes from activated olefins. We intercepted the intermediates prevailing during this transformation and diverted the process for the creation of intricate molecular motifs. We describe an unusual skeletal remodelling of tethered bis-enones to 2,3-epoxy-hexahydrofluoren-9-ones via an “interrupted Corey–Chaykovsky reaction”. The strategy rationally merges the nucleophilic features of sulfur ylides with electronically biased olefins to achieve the regio- and stereoselective synthesis of several new classes of hydrofluorenones. We have demonstrated the synthetic utility of the products in accessing several highly functionalized molecules.
中文翻译:

中断束缚双烯酮的科里-柴可夫斯基反应以获得 2,3-环氧-六氢芴-9-酮
Corey-Chaykovsky 反应通常用于从活化烯烃合成环丙烷。我们截取了这一转化过程中盛行的中间体,并改变了创建复杂分子基序的过程。我们描述了通过“中断的科里-柴可夫斯基反应”将束缚双烯酮转化为 2,3-环氧-六氢芴-9-酮的不寻常的骨架重塑。该策略合理地将硫叶立德的亲核特性与电子偏压烯烃相结合,以实现几种新型氢芴酮的区域和立体选择性合成。我们已经证明了该产品在获得几种高度功能化分子方面的合成效用。
更新日期:2024-05-17
中文翻译:

中断束缚双烯酮的科里-柴可夫斯基反应以获得 2,3-环氧-六氢芴-9-酮
Corey-Chaykovsky 反应通常用于从活化烯烃合成环丙烷。我们截取了这一转化过程中盛行的中间体,并改变了创建复杂分子基序的过程。我们描述了通过“中断的科里-柴可夫斯基反应”将束缚双烯酮转化为 2,3-环氧-六氢芴-9-酮的不寻常的骨架重塑。该策略合理地将硫叶立德的亲核特性与电子偏压烯烃相结合,以实现几种新型氢芴酮的区域和立体选择性合成。我们已经证明了该产品在获得几种高度功能化分子方面的合成效用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号