Synlett ( IF 1.7 ) Pub Date : 2024-05-16 , DOI: 10.1055/s-0043-1763753 Tao Cai 1 , Gaofeng Feng 2 , Yanhua Fu 2 , Chao Zhang 2
|
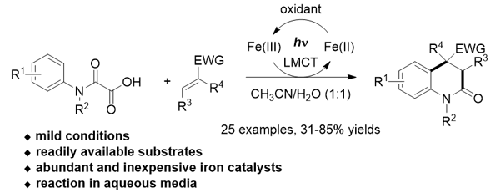
A new protocol for accessing 3,4-dihydroquinolin-2(1H)-ones was established through a sequence of iron-catalyzed photoredox generation of carbamoyl radicals from oxamic acids, addition of the carbamoyl radicals to electron-deficient alkenes, intramolecular cyclization, and aromatization. The process is compatible with a variety of N-phenyloxamic acids and monosubstituted, 1,1-disubstituted, and trisubstituted electron-deficient alkenes. Employing cheap, readily available, and environmentally benign iron as the catalyst, the protocol provides an excellent alternative for synthesis of 3,4-dihydroquinolin-2(1H)-ones.
中文翻译:

通过铁光氧化还原催化生成氨基甲酰基和 3,4-二氢喹啉-2(1H)-酮
通过一系列铁催化光氧化还原从草酰胺酸生成氨基甲酰自由基、将氨基甲酰自由基添加到缺电子烯烃、分子内环化、和芳构化。该工艺与多种N-苯基肟酸和单取代、1,1-二取代和三取代的缺电子烯烃相容。该方案采用廉价、易得且环境友好的铁作为催化剂,为 3,4-二氢喹啉-2(1 H )-酮的合成提供了一种极好的替代方案。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号