Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non-canonical substrate recognition by the human WDR26-CTLH E3 ligase regulates prodrug metabolism
Molecular Cell ( IF 14.5 ) Pub Date : 2024-05-16 , DOI: 10.1016/j.molcel.2024.04.014
Karthik V Gottemukkala 1 , Jakub Chrustowicz 2 , Dawafuti Sherpa 2 , Sara Sepic 1 , Duc Tung Vu 3 , Özge Karayel 3 , Eleftheria C Papadopoulou 1 , Annette Gross 4 , Kenji Schorpp 5 , Susanne von Gronau 2 , Kamyar Hadian 5 , Peter J Murray 6 , Matthias Mann 3 , Brenda A Schulman 1 , Arno F Alpi 2
Molecular Cell ( IF 14.5 ) Pub Date : 2024-05-16 , DOI: 10.1016/j.molcel.2024.04.014
Karthik V Gottemukkala 1 , Jakub Chrustowicz 2 , Dawafuti Sherpa 2 , Sara Sepic 1 , Duc Tung Vu 3 , Özge Karayel 3 , Eleftheria C Papadopoulou 1 , Annette Gross 4 , Kenji Schorpp 5 , Susanne von Gronau 2 , Kamyar Hadian 5 , Peter J Murray 6 , Matthias Mann 3 , Brenda A Schulman 1 , Arno F Alpi 2
Affiliation
|
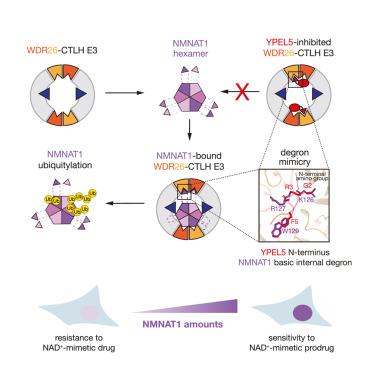
The yeast glucose-induced degradation-deficient (GID) E3 ubiquitin ligase forms a suite of complexes with interchangeable receptors that selectively recruit N-terminal degron motifs of metabolic enzyme substrates. The orthologous higher eukaryotic C-terminal to LisH (CTLH) E3 complex has been proposed to also recognize substrates through an alternative subunit, WDR26, which promotes the formation of supramolecular CTLH E3 assemblies. Here, we discover that human WDR26 binds the metabolic enzyme nicotinamide/nicotinic-acid-mononucleotide-adenylyltransferase 1 (NMNAT1) and mediates its CTLH E3-dependent ubiquitylation independently of canonical GID/CTLH E3-family substrate receptors. The CTLH subunit YPEL5 inhibits NMNAT1 ubiquitylation and cellular turnover by WDR26-CTLH E3, thereby affecting NMNAT1-mediated metabolic activation and cytotoxicity of the prodrug tiazofurin. Cryoelectron microscopy (cryo-EM) structures of NMNAT1- and YPEL5-bound WDR26-CTLH E3 complexes reveal an internal basic degron motif of NMNAT1 essential for targeting by WDR26-CTLH E3 and degron mimicry by YPEL5’s N terminus antagonizing substrate binding. Thus, our data provide a mechanistic understanding of how YPEL5-WDR26-CTLH E3 acts as a modulator of NMNAT1-dependent metabolism.
中文翻译:

人 WDR26-CTLH E3 连接酶的非经典底物识别调节前药代谢
酵母葡萄糖诱导的降解缺陷 (GID) E3 泛素连接酶形成一套具有可互换受体的复合物,这些受体选择性地募集代谢酶底物的 N 末端 degron 基序。LisH 的直系同源高级真核 C 端 (CTLH) E3 复合物也被提出通过替代亚基 WDR26 识别底物,WDR26 促进超分子 CTLH E3 组装体的形成。在这里,我们发现人 WDR26 结合代谢酶烟酰胺/烟酸-单核苷酸-腺苷转移酶 1 (NMNAT1) 并介导其 CTLH E3 依赖性泛素化,独立于经典 GID/CTLH E3 家族底物受体。CTLH 亚基 YPEL5 抑制 WDR26-CTLH E3 的 NMNAT1 泛素化和细胞更新,从而影响 NMNAT1 介导的代谢激活和前药噻唑呋林的细胞毒性。NMNAT1 和 YPEL5 结合的 WDR26-CTLH E3 复合物的冷冻电子显微镜 (cryo-EM) 结构揭示了 NMNAT1 的内部碱性 degron 基序,这对于 WDR26-CTLH E3 靶向和 YPEL5 的 N 末端拮抗底物结合的 degron 模拟至关重要。因此,我们的数据提供了对 YPEL5-WDR26-CTLH E3 如何作为 NMNAT1 依赖性代谢的调节剂的机制理解。
更新日期:2024-05-16
中文翻译:

人 WDR26-CTLH E3 连接酶的非经典底物识别调节前药代谢
酵母葡萄糖诱导的降解缺陷 (GID) E3 泛素连接酶形成一套具有可互换受体的复合物,这些受体选择性地募集代谢酶底物的 N 末端 degron 基序。LisH 的直系同源高级真核 C 端 (CTLH) E3 复合物也被提出通过替代亚基 WDR26 识别底物,WDR26 促进超分子 CTLH E3 组装体的形成。在这里,我们发现人 WDR26 结合代谢酶烟酰胺/烟酸-单核苷酸-腺苷转移酶 1 (NMNAT1) 并介导其 CTLH E3 依赖性泛素化,独立于经典 GID/CTLH E3 家族底物受体。CTLH 亚基 YPEL5 抑制 WDR26-CTLH E3 的 NMNAT1 泛素化和细胞更新,从而影响 NMNAT1 介导的代谢激活和前药噻唑呋林的细胞毒性。NMNAT1 和 YPEL5 结合的 WDR26-CTLH E3 复合物的冷冻电子显微镜 (cryo-EM) 结构揭示了 NMNAT1 的内部碱性 degron 基序,这对于 WDR26-CTLH E3 靶向和 YPEL5 的 N 末端拮抗底物结合的 degron 模拟至关重要。因此,我们的数据提供了对 YPEL5-WDR26-CTLH E3 如何作为 NMNAT1 依赖性代谢的调节剂的机制理解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号