当前位置:
X-MOL 学术
›
Cancer Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Integrative molecular and spatial analysis reveals evolutionary dynamics and tumor-immune interplay of in situ and invasive acral melanoma
Cancer Cell ( IF 48.8 ) Pub Date : 2024-05-16 , DOI: 10.1016/j.ccell.2024.04.012 Hengkang Liu 1 , Jiawen Gao 2 , Mei Feng 3 , Jinghui Cheng 3 , Yuchen Tang 4 , Qi Cao 3 , Ziji Zhao 3 , Ziqiao Meng 3 , Jiarui Zhang 4 , Guohong Zhang 4 , Chong Zhang 4 , Mingming Zhao 4 , Yicen Yan 4 , Yang Wang 4 , Ruidong Xue 1 , Ning Zhang 5 , Hang Li 6
Cancer Cell ( IF 48.8 ) Pub Date : 2024-05-16 , DOI: 10.1016/j.ccell.2024.04.012 Hengkang Liu 1 , Jiawen Gao 2 , Mei Feng 3 , Jinghui Cheng 3 , Yuchen Tang 4 , Qi Cao 3 , Ziji Zhao 3 , Ziqiao Meng 3 , Jiarui Zhang 4 , Guohong Zhang 4 , Chong Zhang 4 , Mingming Zhao 4 , Yicen Yan 4 , Yang Wang 4 , Ruidong Xue 1 , Ning Zhang 5 , Hang Li 6
Affiliation
|
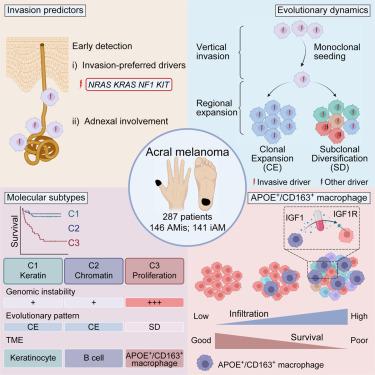
In acral melanoma (AM), progression from (AMis) to invasive AM (iAM) leads to significantly reduced survival. However, evolutionary dynamics during this process remain elusive. Here, we report integrative molecular and spatial characterization of 147 AMs using genomics, bulk and single-cell transcriptomics, and spatial transcriptomics and proteomics. Vertical invasion from AMis to iAM displays an early and monoclonal seeding pattern. The subsequent regional expansion of iAM exhibits two distinct patterns, clonal expansion and subclonal diversification. Notably, molecular subtyping reveals an aggressive iAM subset featured with subclonal diversification, increased epithelial-mesenchymal transition (EMT), and spatial enrichment of APOE/CD163 macrophages. and experiments further demonstrate that APOECD163 macrophages promote tumor EMT via IGF1-IGF1R interaction. Adnexal involvement can predict AMis with higher invasive potential whereas APOE and CD163 serve as prognostic biomarkers for iAM. Altogether, our results provide implications for the early detection and treatment of AM.
中文翻译:

综合分子和空间分析揭示了原位和侵袭性肢端黑色素瘤的进化动力学和肿瘤免疫相互作用
在肢端黑色素瘤 (AM) 中,从 (AMis) 进展为侵袭性 AM (iAM) 会导致生存率显着降低。然而,这个过程中的进化动力学仍然难以捉摸。在这里,我们使用基因组学、本体和单细胞转录组学以及空间转录组学和蛋白质组学报告了 147 个 AM 的综合分子和空间表征。从 AMis 到 iAM 的垂直入侵表现出早期的单克隆播种模式。 iAM 随后的区域扩张表现出两种不同的模式:克隆扩张和亚克隆多样化。值得注意的是,分子亚型分析揭示了一个侵袭性的 iAM 子集,其特征是亚克隆多样化、上皮间质转化 (EMT) 增加以及 APOE/CD163 巨噬细胞的空间富集。实验进一步证明APOECD163巨噬细胞通过IGF1-IGF1R相互作用促进肿瘤EMT。附件受累可以预测具有更高侵袭潜力的 AMis,而 APOE 和 CD163 可作为 iAM 的预后生物标志物。总而言之,我们的结果对 AM 的早期检测和治疗具有重要意义。
更新日期:2024-05-16
中文翻译:

综合分子和空间分析揭示了原位和侵袭性肢端黑色素瘤的进化动力学和肿瘤免疫相互作用
在肢端黑色素瘤 (AM) 中,从 (AMis) 进展为侵袭性 AM (iAM) 会导致生存率显着降低。然而,这个过程中的进化动力学仍然难以捉摸。在这里,我们使用基因组学、本体和单细胞转录组学以及空间转录组学和蛋白质组学报告了 147 个 AM 的综合分子和空间表征。从 AMis 到 iAM 的垂直入侵表现出早期的单克隆播种模式。 iAM 随后的区域扩张表现出两种不同的模式:克隆扩张和亚克隆多样化。值得注意的是,分子亚型分析揭示了一个侵袭性的 iAM 子集,其特征是亚克隆多样化、上皮间质转化 (EMT) 增加以及 APOE/CD163 巨噬细胞的空间富集。实验进一步证明APOECD163巨噬细胞通过IGF1-IGF1R相互作用促进肿瘤EMT。附件受累可以预测具有更高侵袭潜力的 AMis,而 APOE 和 CD163 可作为 iAM 的预后生物标志物。总而言之,我们的结果对 AM 的早期检测和治疗具有重要意义。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号