当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical comparison of fructose with methylglucoside for the production of formate and levulinate catalyzed by Brønsted acids in a methanol solution
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-05-16 , DOI: 10.1039/d4cp01455c Jin-Shan Xiong 1 , Han-Yun Min 1 , Ting Qi 1 , Yin-Sheng Zhang 1 , Chang-Wei Hu 2 , Hua-Qing Yang 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-05-16 , DOI: 10.1039/d4cp01455c Jin-Shan Xiong 1 , Han-Yun Min 1 , Ting Qi 1 , Yin-Sheng Zhang 1 , Chang-Wei Hu 2 , Hua-Qing Yang 1
Affiliation
|
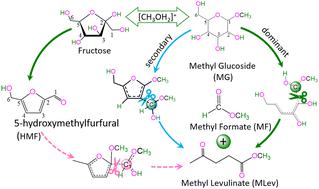
For the conversion of fructose/methylglucoside (MG) into both methyl formate (MF) and methyl levulinate (MLev), the C-source of formate [HCOO]− remains unclear at the molecular level. Herein, reaction mechanisms catalyzed by [CH3OH2]+ in a methanol solution were theoretically investigated at the PBE0/6-311++G(d,p) level. For the conversion of fructose into MF and MLev, the formate [HCOO]− comes from the C1-atom of fructose, in which the rate-determining step lies in the reaction of 5-hydroxymethylfurfural (HMF) with CH3OH to yield MF and MLev. The reaction of fructose with CH3OH kinetically tends to generate HMF intermediates rather than yield (MF + MLev). When MG is dissolved in a methanol solution, its O2, O3, and O4 atoms are closer to the first layer of the solvent than O1, O5, and O6 atoms. For the dehydration of MG with methanol into MF and MLev, the formate [HCOO]− stems from the dominant C1- and secondary C3-atoms of MG. Kinetically, MG is ready to yield (MF + MLev), whereas fructose can induce the reaction to remain at the HMF intermediate, inhibiting the further conversion of HMF with CH3OH into MF and MLev. If MG isomerizes into fructose, the reaction will be more preferable for yielding HMF rather than (MF + MLev).
中文翻译:

甲醇溶液中布朗斯台德酸催化果糖与甲基葡萄糖苷生产甲酸盐和乙酰丙酸盐的理论比较
对于果糖/甲基葡萄糖苷 (MG) 转化为甲酸甲酯 (MF) 和乙酰丙酸甲酯 (MLev) 的过程,甲酸 [HCOO] -的碳源在分子水平上仍不清楚。本文在PBE0/6-311++G(d,p)水平上对甲醇溶液中[CH 3 OH 2 ] +催化的反应机理进行了理论研究。将果糖转化为 MF 和 MLev 时,甲酸盐 [HCOO] -来自果糖的 C1 原子,其中决定速率的步骤在于 5-羟甲基糠醛 (HMF) 与 CH 3 OH 反应生成 MF和MLev。果糖与CH 3 OH的反应动力学倾向于生成HMF中间体而不是产量(MF + MLev)。当MG溶解在甲醇溶液中时,其O2、O3和O4原子比O1、O5和O6原子更靠近溶剂第一层。对于用甲醇将 MG 脱水为 MF 和 MLev,甲酸盐 [HCOO] -源自 MG 的主要 C1 原子和次要 C3 原子。从动力学上讲,MG 已准备好产生 (MF + MLev),而果糖可以诱导反应保留在 HMF 中间体,抑制 HMF 与 CH 3 OH 进一步转化为 MF 和 MLev。如果 MG 异构化为果糖,该反应将更适合生成 HMF,而不是 (MF + MLev)。
更新日期:2024-05-16
中文翻译:

甲醇溶液中布朗斯台德酸催化果糖与甲基葡萄糖苷生产甲酸盐和乙酰丙酸盐的理论比较
对于果糖/甲基葡萄糖苷 (MG) 转化为甲酸甲酯 (MF) 和乙酰丙酸甲酯 (MLev) 的过程,甲酸 [HCOO] -的碳源在分子水平上仍不清楚。本文在PBE0/6-311++G(d,p)水平上对甲醇溶液中[CH 3 OH 2 ] +催化的反应机理进行了理论研究。将果糖转化为 MF 和 MLev 时,甲酸盐 [HCOO] -来自果糖的 C1 原子,其中决定速率的步骤在于 5-羟甲基糠醛 (HMF) 与 CH 3 OH 反应生成 MF和MLev。果糖与CH 3 OH的反应动力学倾向于生成HMF中间体而不是产量(MF + MLev)。当MG溶解在甲醇溶液中时,其O2、O3和O4原子比O1、O5和O6原子更靠近溶剂第一层。对于用甲醇将 MG 脱水为 MF 和 MLev,甲酸盐 [HCOO] -源自 MG 的主要 C1 原子和次要 C3 原子。从动力学上讲,MG 已准备好产生 (MF + MLev),而果糖可以诱导反应保留在 HMF 中间体,抑制 HMF 与 CH 3 OH 进一步转化为 MF 和 MLev。如果 MG 异构化为果糖,该反应将更适合生成 HMF,而不是 (MF + MLev)。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号