当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibitory interactions of the 2,3-dihydro-6,7-dihydroxy-1H-isoindol-1-one scaffold with Bunyavirales cap-snatching endonucleases expose relevant drug design features
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-05-04 , DOI: 10.1016/j.ejmech.2024.116467 Francesca Miglioli 1 , Shindhuja Joel 2 , Matteo Tegoni 1 , Pedro Neira-Pelén 2 , Stephan Günther 2 , Mauro Carcelli 1 , Emilia Fisicaro 3 , Andrea Brancale 4 , Yaiza Fernández-García 2 , Dominga Rogolino 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-05-04 , DOI: 10.1016/j.ejmech.2024.116467 Francesca Miglioli 1 , Shindhuja Joel 2 , Matteo Tegoni 1 , Pedro Neira-Pelén 2 , Stephan Günther 2 , Mauro Carcelli 1 , Emilia Fisicaro 3 , Andrea Brancale 4 , Yaiza Fernández-García 2 , Dominga Rogolino 1
Affiliation
|
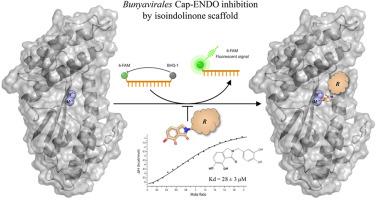
The World Health Organization (WHO) identifies several bunyaviruses as significant threats to global public health security. Developing effective therapies against these viruses is crucial to combat future outbreaks and mitigate their impact on patient outcomes. Here, we report the synthesis of some isoindol-1-one derivatives and explore their inhibitory properties over an indispensable metal-dependent cap-snatching endonuclease (Cap-ENDO) shared among evolutionary divergent bunyaviruses. The compounds suppressed RNA hydrolysis by Cap-ENDOs, with IC values predominantly in the lower μM range. Molecular docking studies revealed the interactions with metal ions to be essential for the 2,3-dihydro-6,7-dihydroxy-1H-isoindol-1-one scaffold activity. Calorimetric analysis uncovered Mn ions to have the highest affinity for sites within the targets, irrespective of aminoacidic variations influencing metal cofactor preferences. Interestingly, spectrophotometric findings unveiled sole dinuclear species formation between the scaffold and Mn. Moreover, the complexation of two Mn ions within the viral enzymes appears to be favourable, as indicated by the binding of compound to TOSV Cap-ENDO (Kd = 28 ± 3 μM). Additionally, the tendency of compound to stabilize His+ more than His- Cap-ENDOs suggests exploitable differences in their catalytic pockets relevant to improving specificity. Collectively, our results underscore the isoindolinone scaffold's potential as a strategic starting point for the design of pan-antibunyavirus drugs.
中文翻译:

2,3-二氢-6,7-二羟基-1H-异吲哚-1-酮支架与布尼亚病毒莱斯帽抢夺核酸内切酶的抑制性相互作用揭示了相关的药物设计特征
世界卫生组织 (WHO) 确定多种布尼亚病毒对全球公共卫生安全构成重大威胁。开发针对这些病毒的有效疗法对于对抗未来的疫情爆发并减轻其对患者治疗结果的影响至关重要。在这里,我们报告了一些异吲哚-1-酮衍生物的合成,并探索了它们对进化趋异的布尼亚病毒共有的一种不可或缺的金属依赖性核酸帽抢夺核酸内切酶(Cap-ENDO)的抑制特性。这些化合物抑制了 Cap-ENDO 的 RNA 水解,IC 值主要在较低的 μM 范围内。分子对接研究表明,与金属离子的相互作用对于 2,3-二氢-6,7-二羟基-1H-异吲哚-1-酮支架活性至关重要。量热分析发现,无论氨基酸变化如何影响金属辅因子偏好,Mn 离子对靶标内的位点具有最高的亲和力。有趣的是,分光光度测定结果揭示了支架和锰之间唯一的双核物种形成。此外,病毒酶内两个 Mn 离子的络合似乎是有利的,如化合物与 TOSV Cap-ENDO 的结合所示(Kd = 28 ± 3 μM)。此外,化合物比 His-Cap-ENDO 更能稳定 His+ 的趋势表明,它们的催化口袋中存在可利用的差异,可提高特异性。总的来说,我们的结果强调了异吲哚酮支架作为泛抗布尼亚病毒药物设计的战略起点的潜力。
更新日期:2024-05-04
中文翻译:

2,3-二氢-6,7-二羟基-1H-异吲哚-1-酮支架与布尼亚病毒莱斯帽抢夺核酸内切酶的抑制性相互作用揭示了相关的药物设计特征
世界卫生组织 (WHO) 确定多种布尼亚病毒对全球公共卫生安全构成重大威胁。开发针对这些病毒的有效疗法对于对抗未来的疫情爆发并减轻其对患者治疗结果的影响至关重要。在这里,我们报告了一些异吲哚-1-酮衍生物的合成,并探索了它们对进化趋异的布尼亚病毒共有的一种不可或缺的金属依赖性核酸帽抢夺核酸内切酶(Cap-ENDO)的抑制特性。这些化合物抑制了 Cap-ENDO 的 RNA 水解,IC 值主要在较低的 μM 范围内。分子对接研究表明,与金属离子的相互作用对于 2,3-二氢-6,7-二羟基-1H-异吲哚-1-酮支架活性至关重要。量热分析发现,无论氨基酸变化如何影响金属辅因子偏好,Mn 离子对靶标内的位点具有最高的亲和力。有趣的是,分光光度测定结果揭示了支架和锰之间唯一的双核物种形成。此外,病毒酶内两个 Mn 离子的络合似乎是有利的,如化合物与 TOSV Cap-ENDO 的结合所示(Kd = 28 ± 3 μM)。此外,化合物比 His-Cap-ENDO 更能稳定 His+ 的趋势表明,它们的催化口袋中存在可利用的差异,可提高特异性。总的来说,我们的结果强调了异吲哚酮支架作为泛抗布尼亚病毒药物设计的战略起点的潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号