当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mesenteric adipose-derived exosomal TINAGL1 enhances intestinal fibrosis in Crohn's Disease via SMAD4
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-05-13 , DOI: 10.1016/j.jare.2024.05.016 Yidong Chen , Junrong Li , Xiaopeng Zhang , Shuang Li , Yiyu Cheng , Xiaoyu Fu , Jiamin Li , Liangru Zhu
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-05-13 , DOI: 10.1016/j.jare.2024.05.016 Yidong Chen , Junrong Li , Xiaopeng Zhang , Shuang Li , Yiyu Cheng , Xiaoyu Fu , Jiamin Li , Liangru Zhu
|
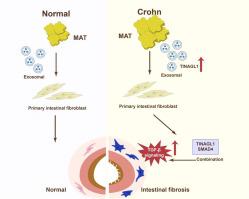
Crohn's Disease (CD) is a chronic inflammatory condition characterized by intestinal fibrosis, severely impacting patient quality of life. The molecular mechanisms driving this fibrosis remain inadequately understood. Recent evidence implicates mesenteric adipose tissue (MAT) in CD pathogenesis, particularly through its exosome secretion, which may influence fibrogenic pathways. Understanding the role of MAT-derived exosomes is crucial for unraveling these molecular processes. This study aims to elucidate the role of MAT-derived exosomes in CD-related intestinal fibrosis. We focus on investigating their molecular composition and the potential impact on fibrosis progression, with an emphasis on identifying novel therapeutic targets. We induced chronic intestinal inflammation in mice using dinitrobenzene sulfonic acid (DNBS), simulating CD-like fibrosis. Exosomes were isolated from DNBS-treated mice (MG) and normal controls (NG) for characterization using electron microscopy and proteomic analysis. Additionally, human colonic fibroblasts were exposed to exosomes from CD patients and healthy individuals, with subsequent assessment of fibrogenesis through proteomic and RNA sequencing analyses. Proteomic analyses revealed a significant activation of the TGF-β signaling pathway in MG-treated mice compared to controls, correlating with enhanced intestinal fibrosis. In vitro experiments demonstrated that colonic fibroblasts exposed to CD patient-derived exosomes exhibited increased fibrogenic activity. Protein docking and co-immunoprecipitation studies suggested a critical interaction between TINAGL1 and SMAD4, enhancing fibrosis. Importantly, in vivo experiments corroborated that recombinant TINAGL1 protein exacerbated DNBS-induced intestinal fibrosis. Our findings highlight the pivotal role of MAT-derived exosomes, particularly those carrying TINAGL1, in the progression of intestinal fibrosis in CD. The involvement of the TGF-β signaling pathway, especially the SMAD4 protein, offers new insights into the molecular mechanisms of CD-related fibrosis and presents potential targets for therapeutic intervention.
中文翻译:

肠系膜脂肪来源的外泌体 TINAGL1 通过 SMAD4 增强克罗恩病的肠道纤维化
克罗恩病(CD)是一种以肠道纤维化为特征的慢性炎症性疾病,严重影响患者的生活质量。驱动这种纤维化的分子机制仍不清楚。最近的证据表明肠系膜脂肪组织 (MAT) 与 CD 发病机制有关,特别是通过其外泌体分泌,这可能会影响纤维形成途径。了解 MAT 衍生的外泌体的作用对于阐明这些分子过程至关重要。本研究旨在阐明 MAT 衍生的外泌体在 CD 相关肠纤维化中的作用。我们专注于研究它们的分子组成以及对纤维化进展的潜在影响,重点是确定新的治疗靶点。我们使用二硝基苯磺酸 (DNBS) 诱导小鼠慢性肠道炎症,模拟 CD 样纤维化。从 DNBS 处理的小鼠 (MG) 和正常对照 (NG) 中分离外泌体,使用电子显微镜和蛋白质组分析进行表征。此外,将人结肠成纤维细胞暴露于来自 CD 患者和健康个体的外泌体,随后通过蛋白质组和 RNA 测序分析评估纤维发生。蛋白质组学分析显示,与对照组相比,MG 治疗小鼠的 TGF-β 信号通路显着激活,与肠纤维化增强相关。体外实验表明,暴露于 CD 患者来源的外泌体的结肠成纤维细胞表现出纤维形成活性增加。蛋白质对接和免疫共沉淀研究表明 TINAGL1 和 SMAD4 之间存在重要的相互作用,可增强纤维化。重要的是,体内实验证实重组 TINAGL1 蛋白会加剧 DNBS 诱导的肠纤维化。 我们的研究结果强调了 MAT 衍生的外泌体,特别是那些携带 TINAGL1 的外泌体,在 CD 肠道纤维化进展中的关键作用。 TGF-β信号通路,尤其是SMAD4蛋白的参与,为CD相关纤维化的分子机制提供了新的见解,并为治疗干预提供了潜在的靶点。
更新日期:2024-05-13
中文翻译:

肠系膜脂肪来源的外泌体 TINAGL1 通过 SMAD4 增强克罗恩病的肠道纤维化
克罗恩病(CD)是一种以肠道纤维化为特征的慢性炎症性疾病,严重影响患者的生活质量。驱动这种纤维化的分子机制仍不清楚。最近的证据表明肠系膜脂肪组织 (MAT) 与 CD 发病机制有关,特别是通过其外泌体分泌,这可能会影响纤维形成途径。了解 MAT 衍生的外泌体的作用对于阐明这些分子过程至关重要。本研究旨在阐明 MAT 衍生的外泌体在 CD 相关肠纤维化中的作用。我们专注于研究它们的分子组成以及对纤维化进展的潜在影响,重点是确定新的治疗靶点。我们使用二硝基苯磺酸 (DNBS) 诱导小鼠慢性肠道炎症,模拟 CD 样纤维化。从 DNBS 处理的小鼠 (MG) 和正常对照 (NG) 中分离外泌体,使用电子显微镜和蛋白质组分析进行表征。此外,将人结肠成纤维细胞暴露于来自 CD 患者和健康个体的外泌体,随后通过蛋白质组和 RNA 测序分析评估纤维发生。蛋白质组学分析显示,与对照组相比,MG 治疗小鼠的 TGF-β 信号通路显着激活,与肠纤维化增强相关。体外实验表明,暴露于 CD 患者来源的外泌体的结肠成纤维细胞表现出纤维形成活性增加。蛋白质对接和免疫共沉淀研究表明 TINAGL1 和 SMAD4 之间存在重要的相互作用,可增强纤维化。重要的是,体内实验证实重组 TINAGL1 蛋白会加剧 DNBS 诱导的肠纤维化。 我们的研究结果强调了 MAT 衍生的外泌体,特别是那些携带 TINAGL1 的外泌体,在 CD 肠道纤维化进展中的关键作用。 TGF-β信号通路,尤其是SMAD4蛋白的参与,为CD相关纤维化的分子机制提供了新的见解,并为治疗干预提供了潜在的靶点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号