当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Single-nucleus transcriptional and chromatin accessible profiles reveal critical cell types and molecular architecture underlying chicken sex determination
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-05-10 , DOI: 10.1016/j.jare.2024.05.007 Jianbo Li 1 , Xiuan Zhang 1 , Xiqiong Wang 1 , Zhen Wang 2 , Xingzheng Li 2 , Jiangxia Zheng 1 , Junying Li 1 , Guiyun Xu 1 , Congjiao Sun 1 , Guoqiang Yi 2 , Ning Yang 1
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-05-10 , DOI: 10.1016/j.jare.2024.05.007 Jianbo Li 1 , Xiuan Zhang 1 , Xiqiong Wang 1 , Zhen Wang 2 , Xingzheng Li 2 , Jiangxia Zheng 1 , Junying Li 1 , Guiyun Xu 1 , Congjiao Sun 1 , Guoqiang Yi 2 , Ning Yang 1
Affiliation
|
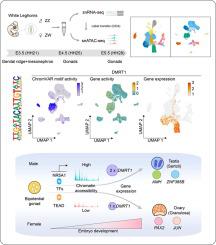
Understanding the sex determination mechanisms in birds has great significance for the biological sciences and production in the poultry industry. Sex determination in chickens is a complex process that involves fate decisions of supporting cells such as granulosa or Sertoli cells. However, a systematic understanding of the genetic regulation and cell commitment process underlying sex determination in chickens is still lacking. We aimed to dissect the molecular characteristics associated with sex determination in the gonads of chicken embryos. Single-nucleus RNA-seq (snRNA-seq) and ATAC-seq (snATAC-seq) analysis were conducted on the gonads of female and male chickens at embryonic day 3.5 (E3.5), E4.5, and E5.5. Here, we provided a time-course transcriptional and chromatin accessible profiling of gonads during chicken sex determination at single-cell resolution. We uncovered differences in cell composition and developmental trajectories between female and male gonads and found that the divergence of transcription and accessibility in gonadal cells first emerged at E5.5. Furthermore, we revealed key cell-type-specific transcription factors (TFs) and regulatory networks that drive lineage commitment. Sex determination signaling pathways, dominated by BMP signaling, are preferentially activated in males during gonadal development. Further pseudotime analysis of the supporting cells indicated that granulosa cells were regulated mainly by the TEAD gene family and that Sertoli cells were driven by the DMRT1 regulons. Cross-species analysis suggested high conservation of both cell types and cell-lineage-specific TFs across the six vertebrates. Overall, our study will contribute to accelerating the development of sex manipulation technology in the poultry industry and the application of chickens as a unique model for studying cell fate decisions.
中文翻译:

单核转录和染色质可及图谱揭示了鸡性别决定的关键细胞类型和分子结构
了解鸟类的性别决定机制对于生物科学和家禽业生产具有重要意义。鸡的性别决定是一个复杂的过程,涉及支持细胞(如颗粒细胞或支持细胞)的命运决定。然而,仍然缺乏对鸡性别决定背后的遗传调控和细胞定型过程的系统了解。我们的目的是剖析与鸡胚胎性腺性别决定相关的分子特征。对胚胎第 3.5 天 (E3.5)、E4.5 和 E5.5 的雌性和雄性鸡的性腺进行单核 RNA-seq (snRNA-seq) 和 ATAC-seq (snATAC-seq) 分析。在这里,我们以单细胞分辨率提供了鸡性别测定过程中性腺的时间进程转录和染色质可访问分析。我们发现了女性和男性性腺之间细胞组成和发育轨迹的差异,并发现性腺细胞转录和可及性的差异首先出现在E5.5。此外,我们还揭示了推动谱系定型的关键细胞类型特异性转录因子(TF)和调控网络。以 BMP 信号为主的性别决定信号通路在男性性腺发育过程中优先被激活。对支持细胞的进一步伪时间分析表明,颗粒细胞主要受 TEAD 基因家族调节,支持细胞受 DMRT1 调节子驱动。跨物种分析表明,六种脊椎动物的细胞类型和细胞谱系特异性转录因子均高度保守。 总的来说,我们的研究将有助于加速家禽业性别操纵技术的发展,以及将鸡作为研究细胞命运决定的独特模型的应用。
更新日期:2024-05-10
中文翻译:

单核转录和染色质可及图谱揭示了鸡性别决定的关键细胞类型和分子结构
了解鸟类的性别决定机制对于生物科学和家禽业生产具有重要意义。鸡的性别决定是一个复杂的过程,涉及支持细胞(如颗粒细胞或支持细胞)的命运决定。然而,仍然缺乏对鸡性别决定背后的遗传调控和细胞定型过程的系统了解。我们的目的是剖析与鸡胚胎性腺性别决定相关的分子特征。对胚胎第 3.5 天 (E3.5)、E4.5 和 E5.5 的雌性和雄性鸡的性腺进行单核 RNA-seq (snRNA-seq) 和 ATAC-seq (snATAC-seq) 分析。在这里,我们以单细胞分辨率提供了鸡性别测定过程中性腺的时间进程转录和染色质可访问分析。我们发现了女性和男性性腺之间细胞组成和发育轨迹的差异,并发现性腺细胞转录和可及性的差异首先出现在E5.5。此外,我们还揭示了推动谱系定型的关键细胞类型特异性转录因子(TF)和调控网络。以 BMP 信号为主的性别决定信号通路在男性性腺发育过程中优先被激活。对支持细胞的进一步伪时间分析表明,颗粒细胞主要受 TEAD 基因家族调节,支持细胞受 DMRT1 调节子驱动。跨物种分析表明,六种脊椎动物的细胞类型和细胞谱系特异性转录因子均高度保守。 总的来说,我们的研究将有助于加速家禽业性别操纵技术的发展,以及将鸡作为研究细胞命运决定的独特模型的应用。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号